Fully humanized anti-CD20 recombinant antibody
A recombinant antibody, fully human technology, applied in recombinant DNA technology, antibody mimic/scaffold, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problem of lack of Fc segment function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Expression and Identification of CD20 Antigen
[0032] The CD20 eukaryotic expression vector was constructed to highly express CD20 protein in HEK293T cells.
[0033] Methods: Using the total RNA of human peripheral blood mononuclear cells (PBMC) as a template, the CD20 gene was amplified by reverse transcription PCR and inserted into the eukaryotic expression vector pCMV3-C-His. After preliminary verification by PCR, it was verified by sequencing. The recombinant plasmid was transiently transfected into HEK293T cells for protein expression, and the expression of CD20 protein was detected by ELISA and Western blot, and the expression conditions were optimized.
[0034] The specific experimental steps of this example have been disclosed in this document: "Shi Mei, Nian Siji, Gao Yan, Wu Yuchuan, Song Zhangyong, Wang Mingxue, Yuan Qing. High expression of soluble CD20 in HEK293T cells[J]. Cellular and Molecular Immunology Journal of Science, 2018, 34(10):870-8...
Embodiment 2
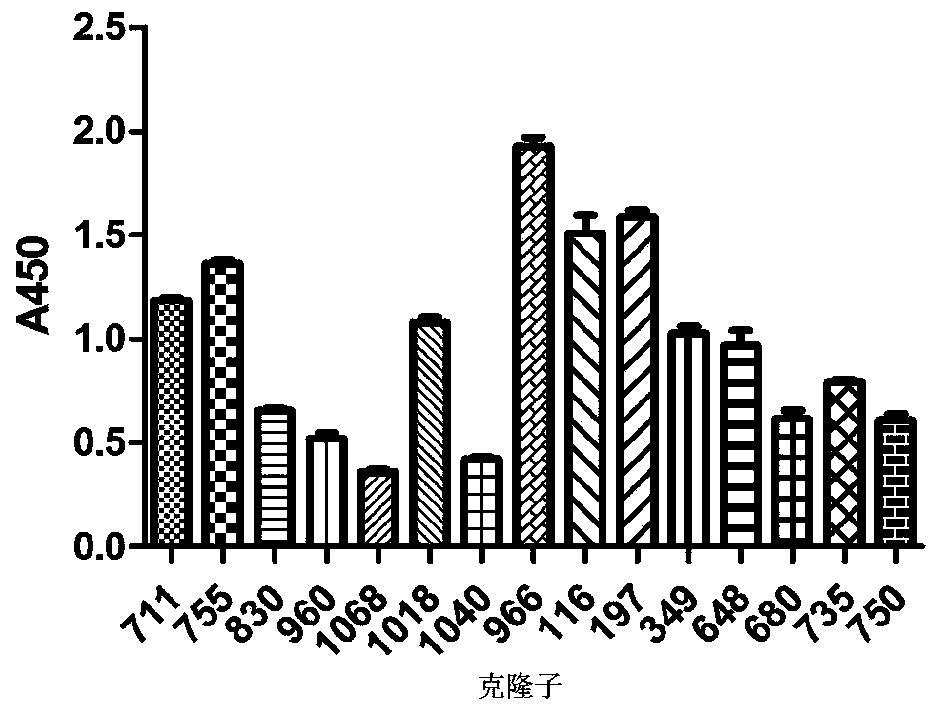
[0037] Example 2: Anti-CD20 single-chain antibody screening
[0038] 1. Biotinylation of CD20 protein
[0039] Take about 40 μl of 50 μg / ml CD20 protein (purchased from China Sino Biological Company) dissolved in 1×PBS for use; weigh 1 mg biotin biotin, add 160 μl ddH 2 O was dissolved, and 1 μl was added to the aforementioned 40 μl CD20 protein solution, and incubated on ice for 2 hours or at room temperature at 25°C for 1 hour. The biotinylated protein was added to the desalting column, and the biotinylated protein was collected by centrifugation at 1000g for 2 minutes and stored at -80°C.
[0040] 2. Phage library screening
[0041] Our laboratory has previously constructed a natural fully human phage antibody library with a library capacity of 2.5×10 8 . Take 5×10 11 TU scFv antibody library phages were blocked with blocking solution (6%BSA / 1×PBST) for 1h at room temperature; biotinylated CD20 protein was added and incubated at 37°C for 2h to form CD20 protein-specif...
Embodiment 3
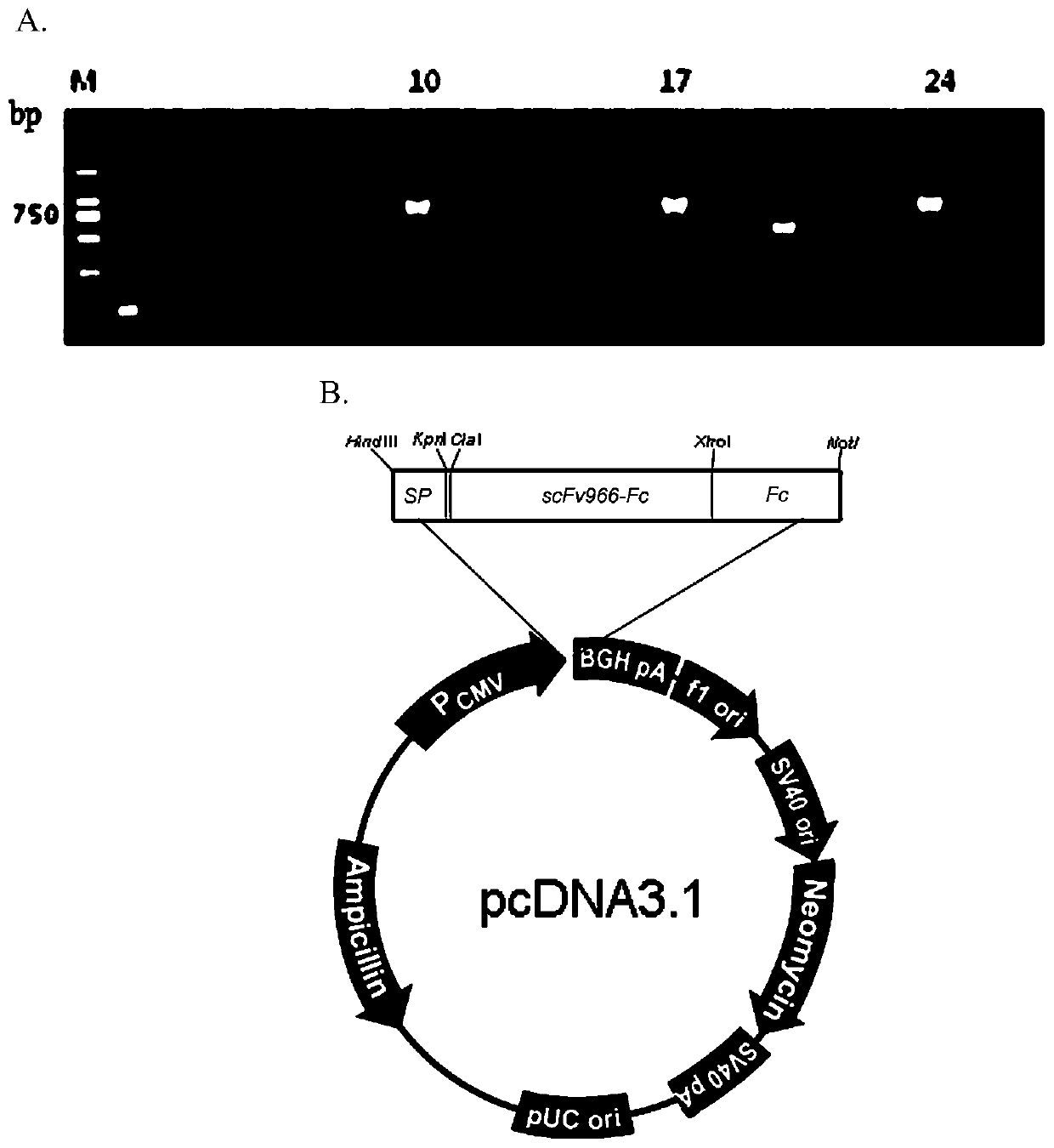
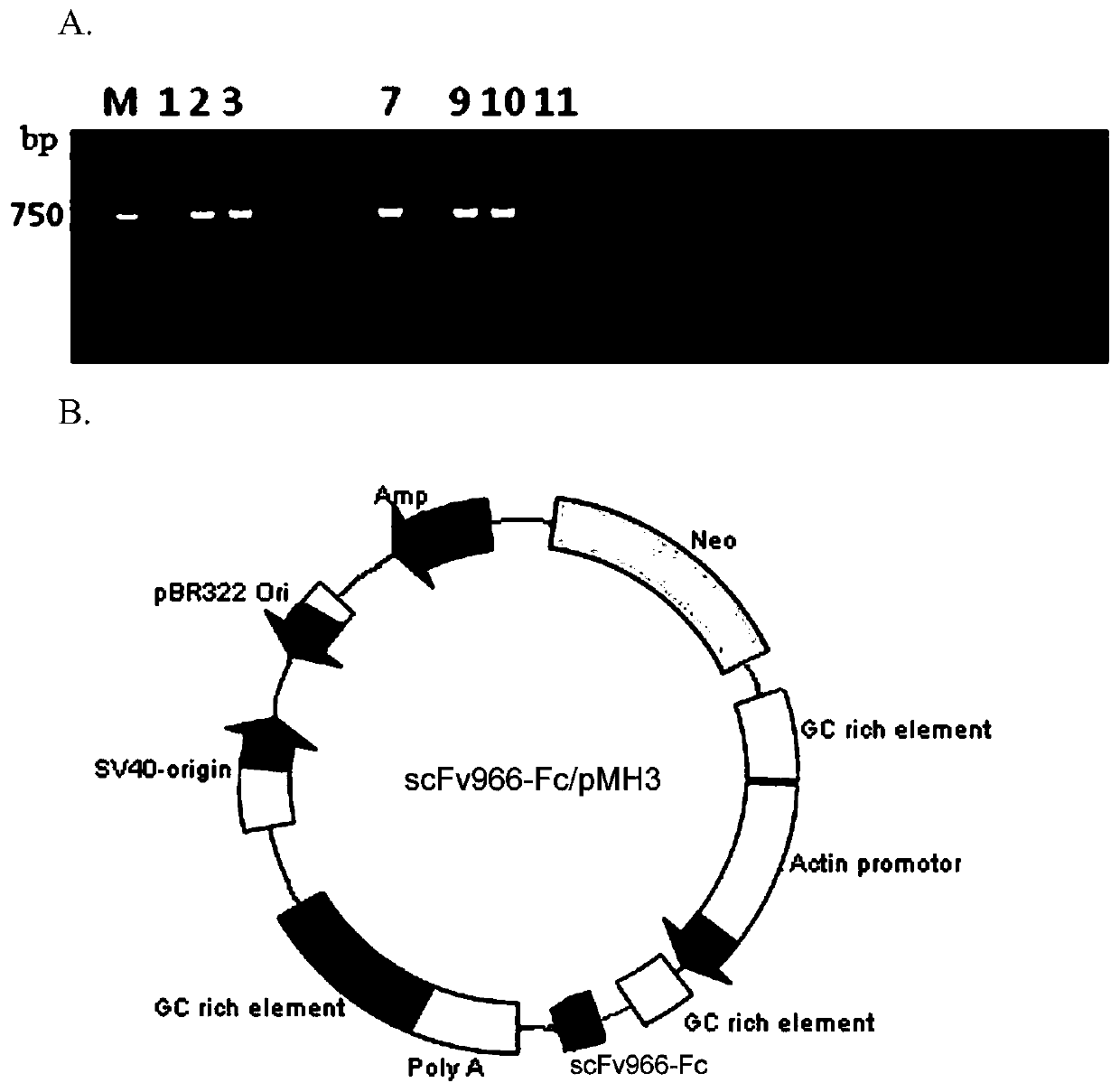
[0048] Example 3. Construction and expression of anti-CD20 scFv966-Fc recombinant expression vector
[0049] 1. Construction of sp-scFv966-Fc / pcDNA3.1 recombinant expression vector
[0050] 1. PCR amplification of scFv fragments
[0051] The scFv966 fragment was obtained by PCR amplification with high-fidelity Pfu DNA polymerase using scFv966-F (containing ClaⅠ restriction site) as the upstream primer and scFv966-R as the downstream primer (containing XhoⅠ restriction site). The sequence of the upstream primer scFv966-F is: 5'-CCCATCGATATGGGCCCAGGTGCAGCT-3' (SEQ ID No: 4), and the sequence of the downstream primer scFv966-R is: 5'-CCGCTCGAGACCTAGGACGGTCAGCTTGG-3' (SEQ ID No: 5). The 20μl PCR reaction system is: 10×Pfu PCRBuffer 2μl, 2.5mM dNTPs 1.6μl (final concentration 200μM), scFv966-F (10μM) 1μl, scFv966-R (10μM) 1μl, 2.5U ClonedPfu DNApolymerase 0.4μl, scFv966 DNA template 1μl (50ng / μl), add ddH 2 0 to 20 μl. PCR amplification conditions: 94°C for 2min; 98°C for 30sec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com