Use of IFI44L gene and PI3 gene in preparation of diagnostic bacterial/viral infection kit
A virus infection and kit technology, applied in the field of biomedicine, can solve problems such as specificity and sensitivity not meeting clinical needs, missed diagnosis, misdiagnosis of patients, etc., and achieve the effect of being conducive to early diagnosis, high sensitivity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
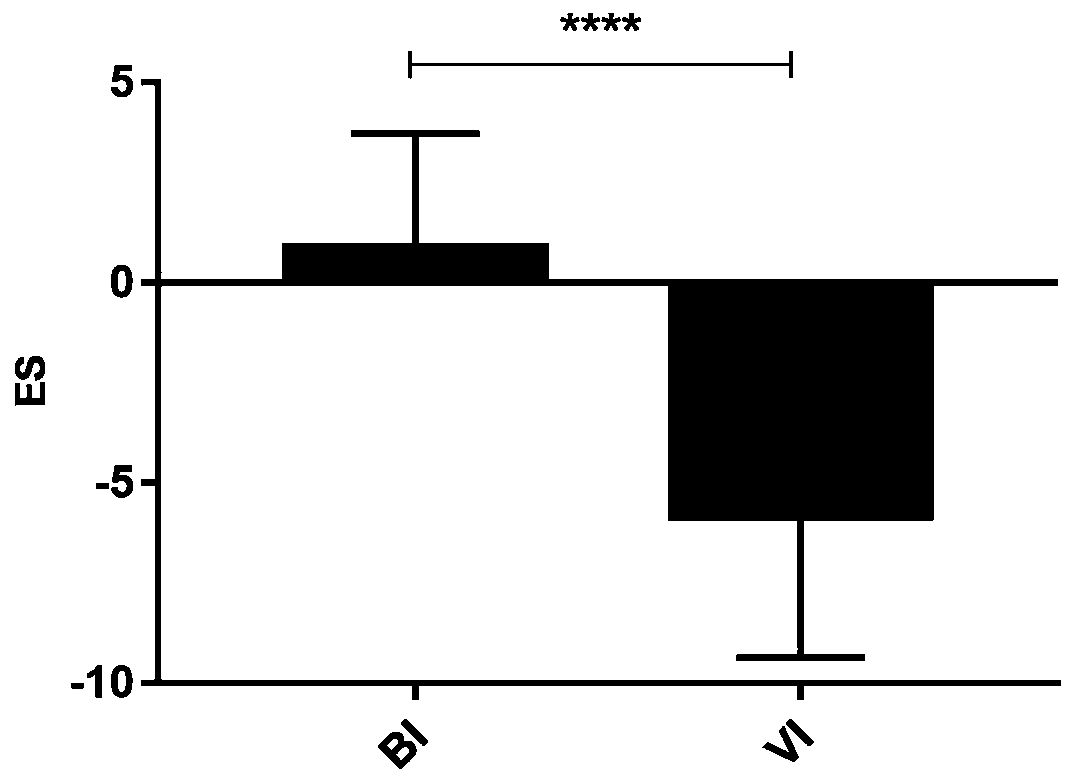
[0040] Example 1. Screening for differentially expressed genes in the state of bacterial infection and viral infection
[0041] 1. Clinical research object:
[0042] Two groups of patients with definite bacterial infection and definite viral infection in febrile period were selected, with 30 patients in each group.
[0043] Diagnostic criteria:
[0044] (1) The definite bacterial infection group refers to patients with bacterial infection confirmed by culture;
[0045] (2) The definite viral infection group includes only patients with viral infection confirmed by culture, molecular or immunofluorescence detection, and no features of coexisting bacterial infection.
[0046] Exclusion criteria: patients with bone marrow transplantation, immunodeficiency or immunosuppressive therapy that may affect gene expression.
[0047] All research subjects signed the informed consent.
[0048] 2. Sample collection
[0049] During the febrile period, blood samples were drawn at the same...
Embodiment 2
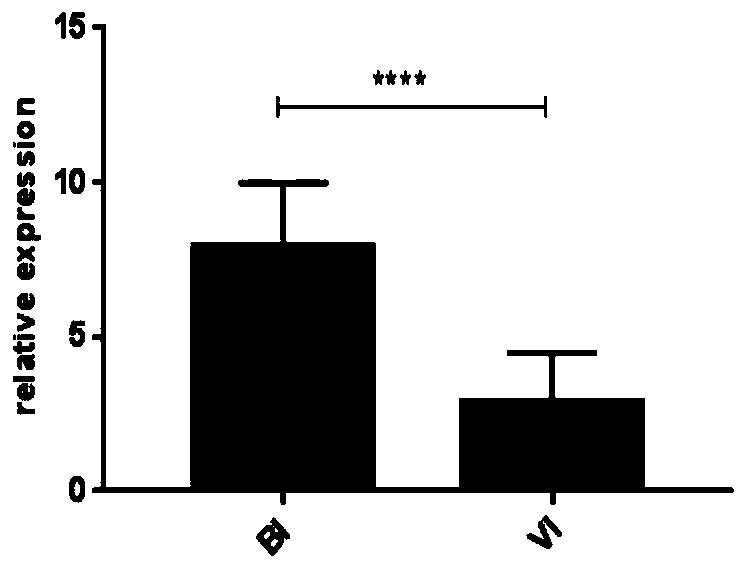
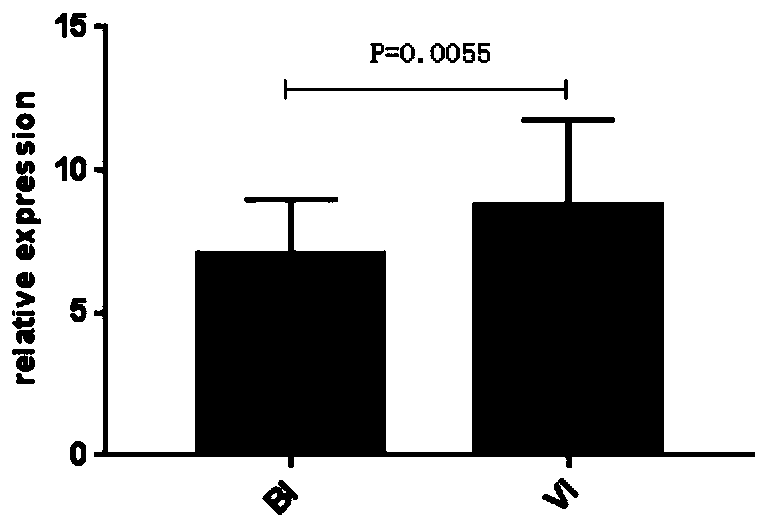
[0084] Embodiment 2, qPCR verifies the relation of IFI44L gene and PI3 gene and bacterial infection, virus infection disease
[0085] 1. Research object:
[0086] 35 patients with confirmed bacterial infection and 36 patients with confirmed viral infection were selected during the febrile period.
[0087] Diagnostic criteria:
[0088] (1) The definite bacterial infection group refers to patients with bacterial infection confirmed by culture;
[0089] (2) The definite viral infection group includes only patients with viral infection confirmed by culture, molecular or immunofluorescence detection, and no features of coexisting bacterial infection.
[0090] 2. Total RNA extraction from blood
[0091]Use the UNIQ-10 Column Trizol Total RNA Extraction Kit to extract the total RNA from the blood of the above-mentioned patients with fever. The extraction steps are as follows:
[0092] (1) EDTA anticoagulant blood 2-3mL whole blood, immediately use RNase-free EP tubes to aliquot a...
Embodiment 3
[0117] Example 3, qPCR re-verified the relationship between IFI44L gene and PI3 gene and bacterial infection and viral infection
[0118] 1. Research object:
[0119] 42 patients with confirmed bacterial infection, 56 patients with confirmed viral infection and 31 healthy controls (HC) were selected.
[0120] Diagnostic criteria:
[0121] (1) The definite bacterial infection group refers to patients with bacterial infection confirmed by culture;
[0122] (2) The definite viral infection group includes only patients with viral infection confirmed by culture, molecular or immunofluorescence detection, and no features of coexisting bacterial infection.
[0123] 2. Total RNA extraction from blood
[0124] Use the UNIQ-10 Column Trizol Total RNA Extraction Kit to extract the total RNA from the blood of the above-mentioned patients with fever. The extraction steps are as follows:
[0125] (1) EDTA anticoagulant blood 2-3mL whole blood, immediately use RNase-free EP tubes to aliq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com