Pneumococcal conjugate vaccine formulations
一种蛋白缀合物、制剂的技术,应用在疫苗、多价疫苗、抗细菌药等方向,能够解决肺炎球菌多糖应答差等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
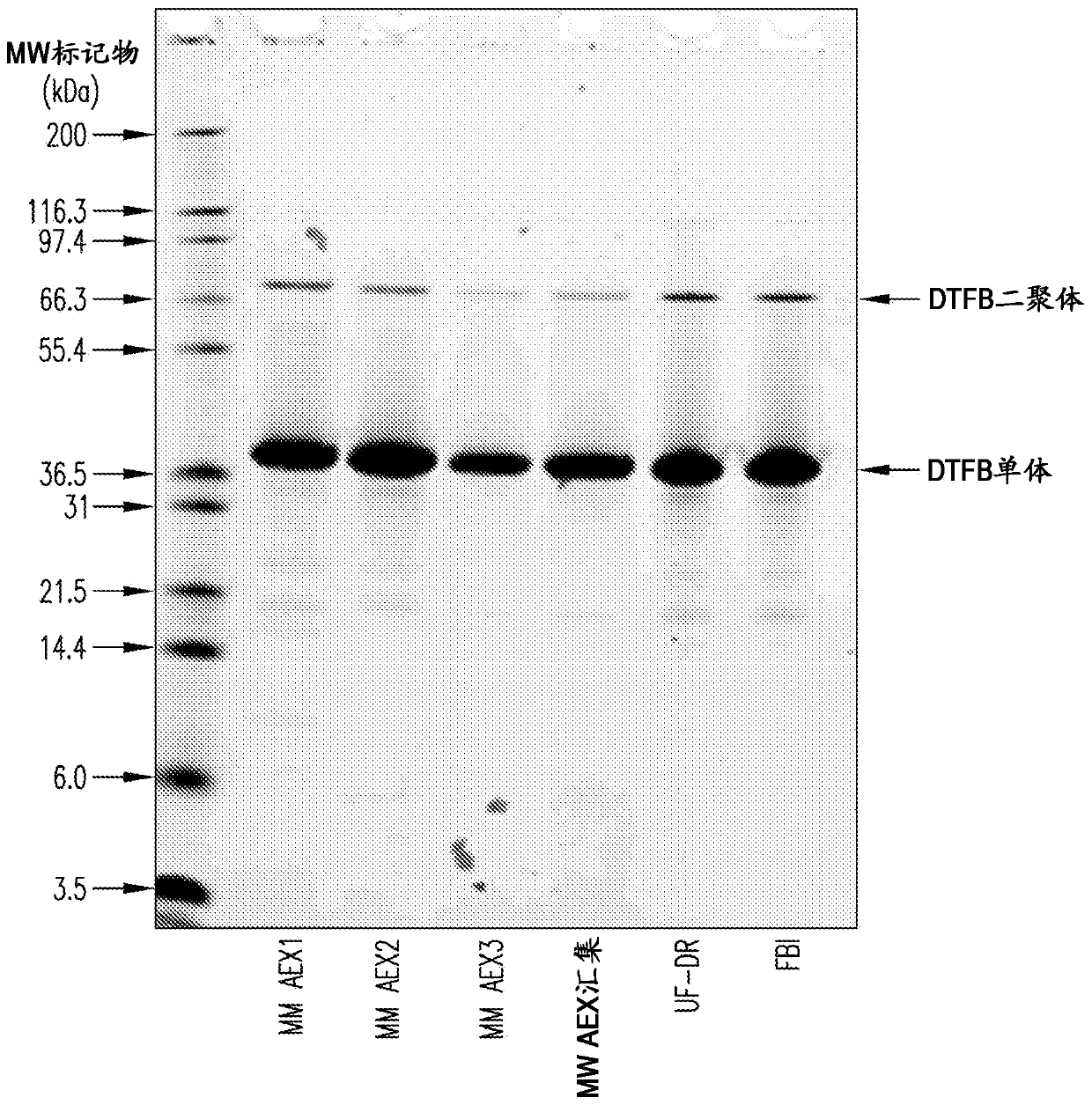
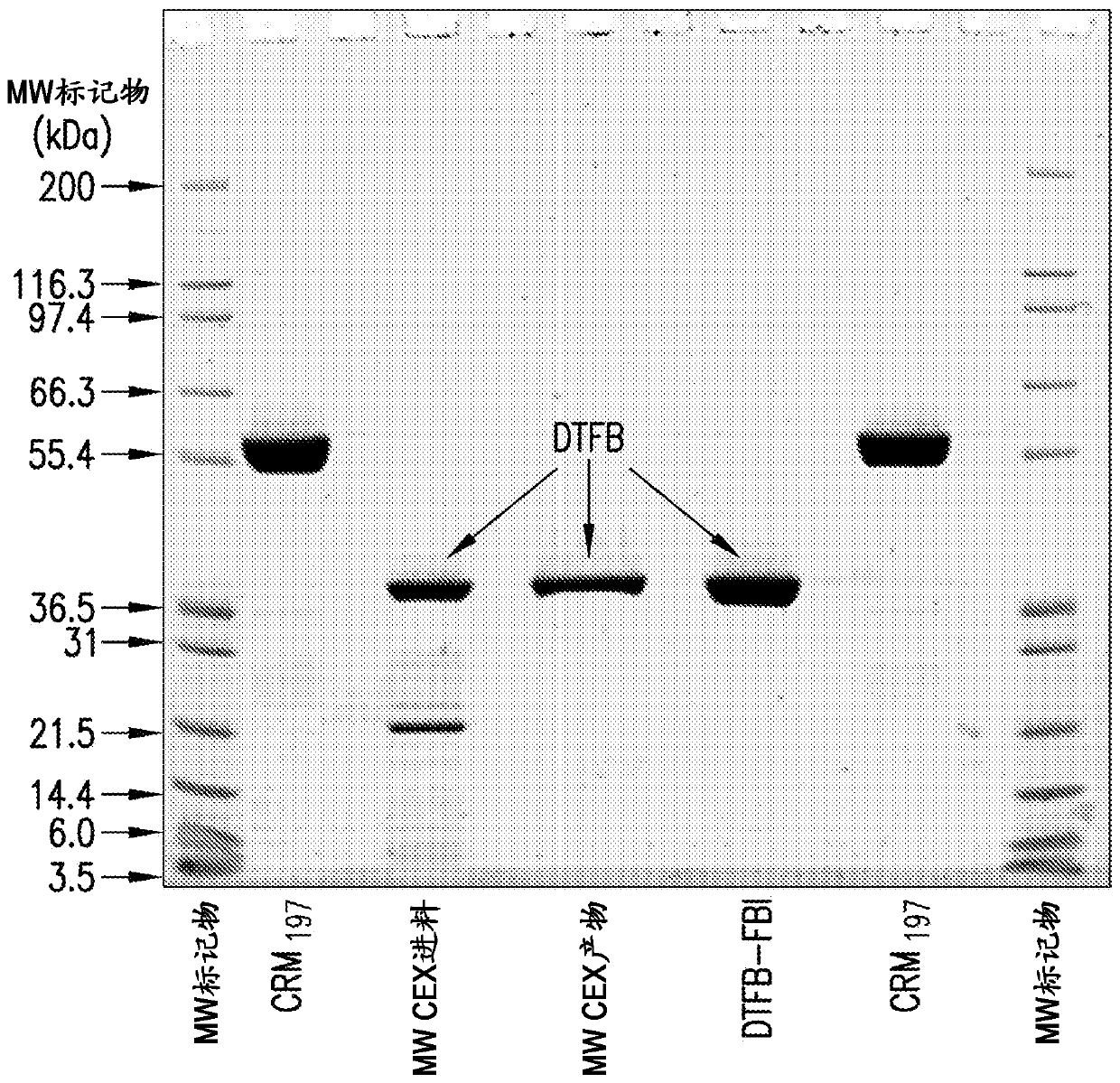
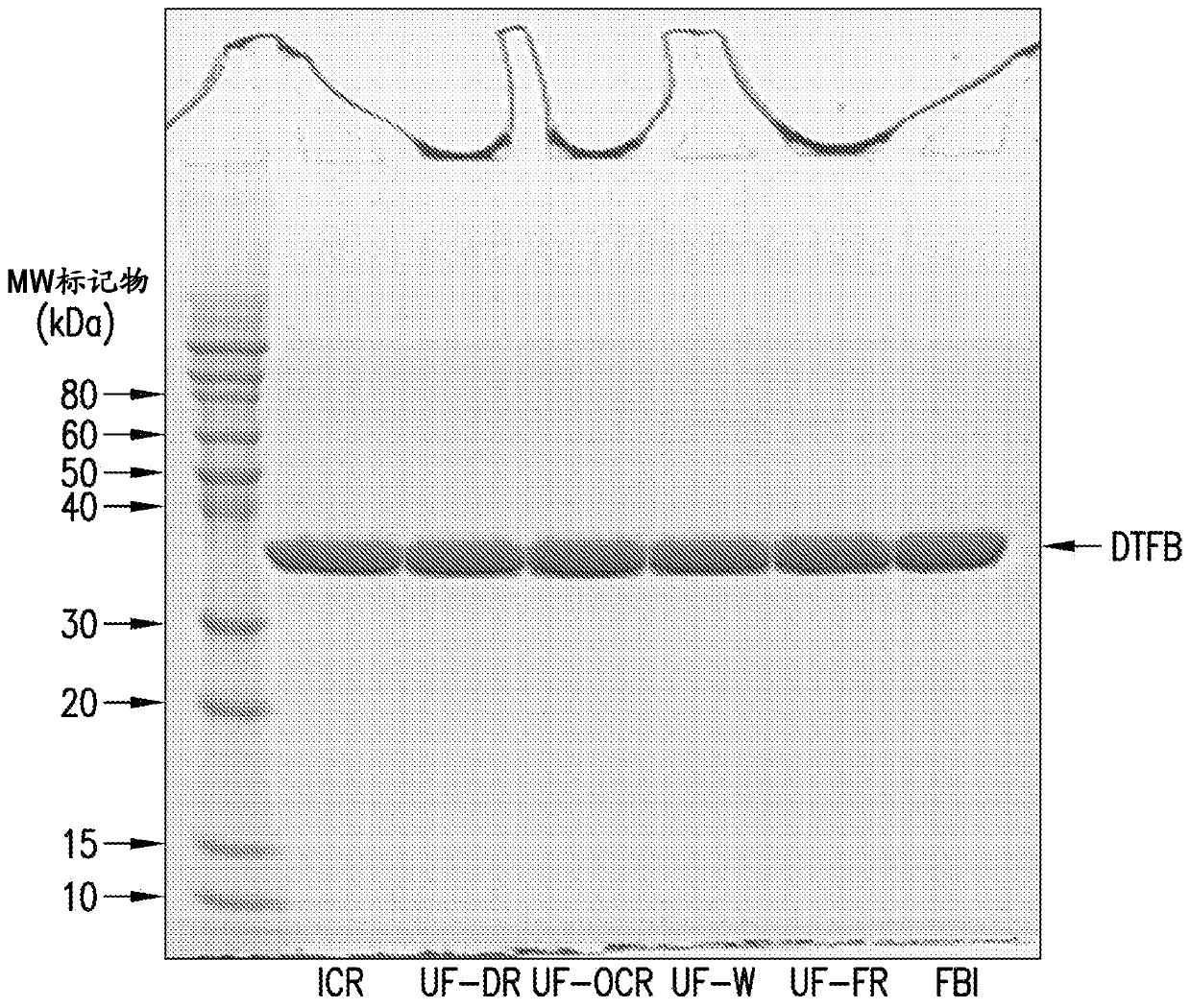
[0142] Embodiment 1: the preparation of DTFB carrier protein
[0143] Using mixed-mode anion-exchange chromatography for the preparation of DTFB
[0144] Use a 1:500 molar ratio of trypsin to CRM 197 Purified CRM obtained by expression in Pseudomonas fluorescens as described 197 (See International Patent Application Publication No. WO2012 / 173876A1) Digested with recombinant trypsin. Dithiothreitol (DTT) in 50 mM Tris, pH 8 was then added to a final concentration of 5 mM for 30 minutes at about 22°C to reduce the disulfide bond between the cleaved A and B fragments by proteolysis.
[0145] The digestion reaction was then loaded onto a mixed-mode anion-exchange column (Capto TM Adhere, GE Healthcare). The column was washed with 50 mM Tris, pH 8, and the DTFB product was eluted with a gradient of 0.45M to 0.65M sodium chloride in 50 mM Tris, pH 8. The product was concentrated and diafiltered against 10 mM potassium phosphate, pH 8, using a 5 kDa nominal molecular weight cut...
Embodiment 2
[0163] Embodiment 2: Preparation of Streptococcus pneumoniae capsular polysaccharide
[0164] Methods for culturing pneumococci are well known in the art. See, eg, Chase, 1967, Methods of Immunology and Immunochemistry 1:52. Methods for preparing pneumococcal capsular polysaccharides are also well known in the art. See eg European Patent No. EP0497524. Isolates of pneumococcal subtypes are available from the American Type Culture Collection (Manassas, VA). Bacteria were identified as encapsulated, non-motile, Gram-positive, lancet-shaped diplococci that were alpha-hemolytic on blood agar. Subtypes can be distinguished on the basis of the Quelling reaction using specific antisera. See, eg, US Patent No. 5,847,112.
[0165] Cell banks representing each S. pneumoniae serotype of interest were obtained in frozen vials from the Merck Culture Collection (Rahway, NJ). The thawed seed culture was transferred to a seed fermenter containing pre-sterilized growth medium suitable fo...
Embodiment 3
[0167] Example 3: Conjugation of polysaccharides to DTFB carrier proteins using reductive amination in aqueous solution
[0168] Preparation of serotype 3-DTFB (ST3-DTFB) conjugates for mouse immunogenicity studies Purified serotype 3 polysaccharides obtained as described in Example 2 were dissolved in water. Ps were size reduced to an average molecular weight of approximately 200 kDa using a probe sonicator with the samples cooled in ice. Sonicated samples were filtered through 0.2 micron and stored at 2-8°C. The polysaccharide solution was concentrated by diafiltration against a 30 kDa NMWCO tangential flow filtration membrane.
[0169] Sodium metaperiodate oxidation was used to prepare polysaccharides for conjugation (see Anderson et al., 1986, J. Immunol. 137:1181-1186; and US Patent Application Publication No. US20110195086). A 100 mM sodium metaperiodate solution was added to the polysaccharide solution in 50 mM sodium acetate. The samples were mixed in the dark for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com