Method for joint production of industrial-grade potassium dihydrogen phosphate and feed-grade calcium hydrogen phosphate by wet-process phosphoric acid
A technology of high-grade potassium dihydrogen phosphate and wet-process phosphoric acid, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of difficult product purity, low purity of calcium hydrogen phosphate, and low economic value, and achieve good results. Social benefit and economic benefit, improvement of utilization value, effect of low quality requirement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
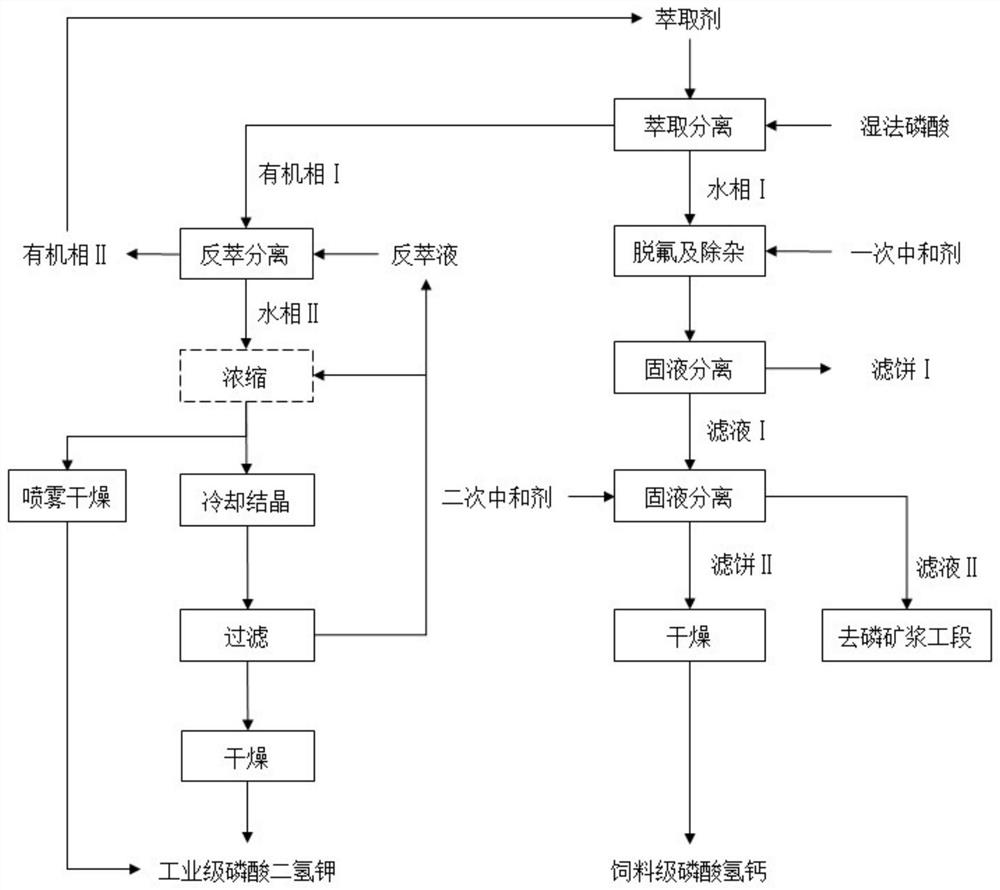
[0068] a.P 2 o 5 Concentration is 24.18wt% 1000g wet-process phosphoric acid, use tributyl phosphate TBP as extractant, carry out extraction reaction according to the volume ratio of wet-process phosphoric acid and extractant is 1:3, control reaction temperature to be 60 ℃, reaction time 30min, Stand still and separate phases to obtain organic phase I and aqueous phase I.
[0069] b. The organic phase I obtained in step a and 635.7 g of a potassium hydroxide solution with a mass fraction of 15 wt % are subjected to a stripping reaction, and the amount added is based on the potassium hydroxide in the stripping agent and the P in the organic phase I. 2 o 5 The molar ratio is calculated as 2:1, the reaction temperature is controlled at 60°C, the reaction time is 30min, and the phases are separated by standing to obtain the organic phase II and the aqueous phase II; wherein, the organic phase II is the regenerative extractant, and the aqueous phase II is the phosphoric acid diph...
Embodiment 2
[0078] a.P 2 o 5 Concentration is 1000g wet-process phosphoric acid of 35.72wt%, adopt n-butanol as extractant, carry out extraction reaction according to the volume ratio of wet-process phosphoric acid and extractant is 1:3, control reaction temperature to be 60 ℃, stand still and separate phases, obtain Organic phase I and aqueous phase I.
[0079] b. The organic phase I obtained in step a and 1760.9 g of potassium bicarbonate solution with a mass fraction of 20wt% are subjected to stripping reaction, and the amount added is based on the potassium bicarbonate in the stripping agent and the P in the organic phase I. 2 o 5 The molar ratio is calculated as 2:1, the reaction temperature is controlled at 60°C, the reaction time is 30min, and the phases are separated by standing to obtain the organic phase II and the aqueous phase II; wherein, the organic phase II is the regenerative extractant, and the aqueous phase II is the phosphoric acid diphosphate A crude solution of pot...
Embodiment 3
[0088] a.P 2 o 5 1000g of wet-process phosphoric acid with a concentration of 46.25wt%, using diisopropyl ether as the extractant, carrying out the extraction reaction at a volume ratio of wet-process phosphoric acid and extractant of 1:3, controlling the reaction temperature to 60°C, and standing for phase separation. An organic phase I and an aqueous phase I were obtained.
[0089] B, the organic phase I obtained in the step a and the potassium carbonate solution 1797.9g of mass fraction 15wt% carry out stripping reaction, add the potassium carbonate in stripping agent and the P in organic phase I 2 o 5 The molar ratio is calculated as 1:1, the reaction temperature is controlled at 60°C, the reaction time is 30min, and the phases are separated by standing to obtain the organic phase II and the aqueous phase II; wherein, the organic phase II is the regenerative extractant, and the aqueous phase II is the phosphoric acid diphosphate A crude solution of potassium hydrogen. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com