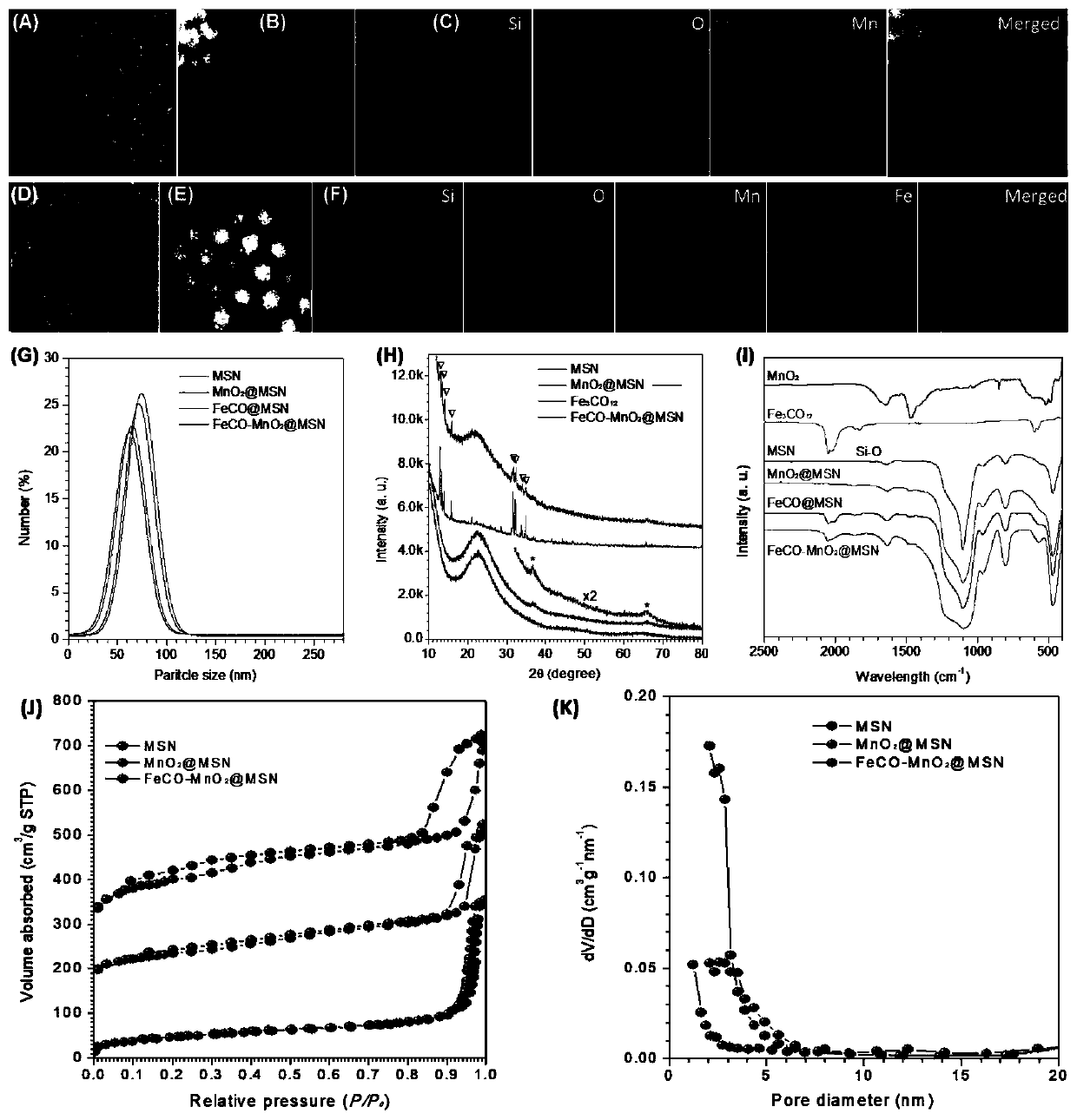
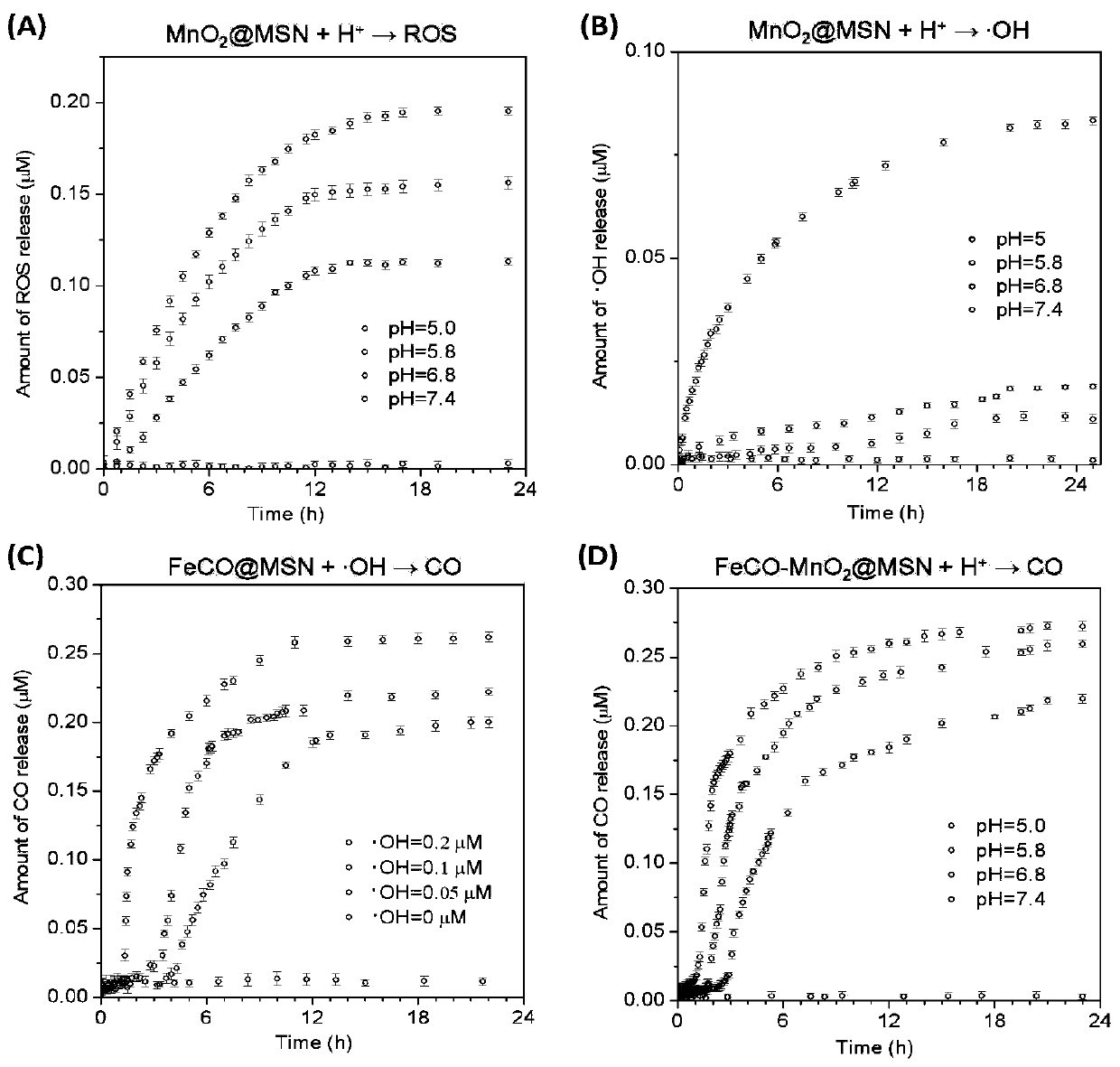
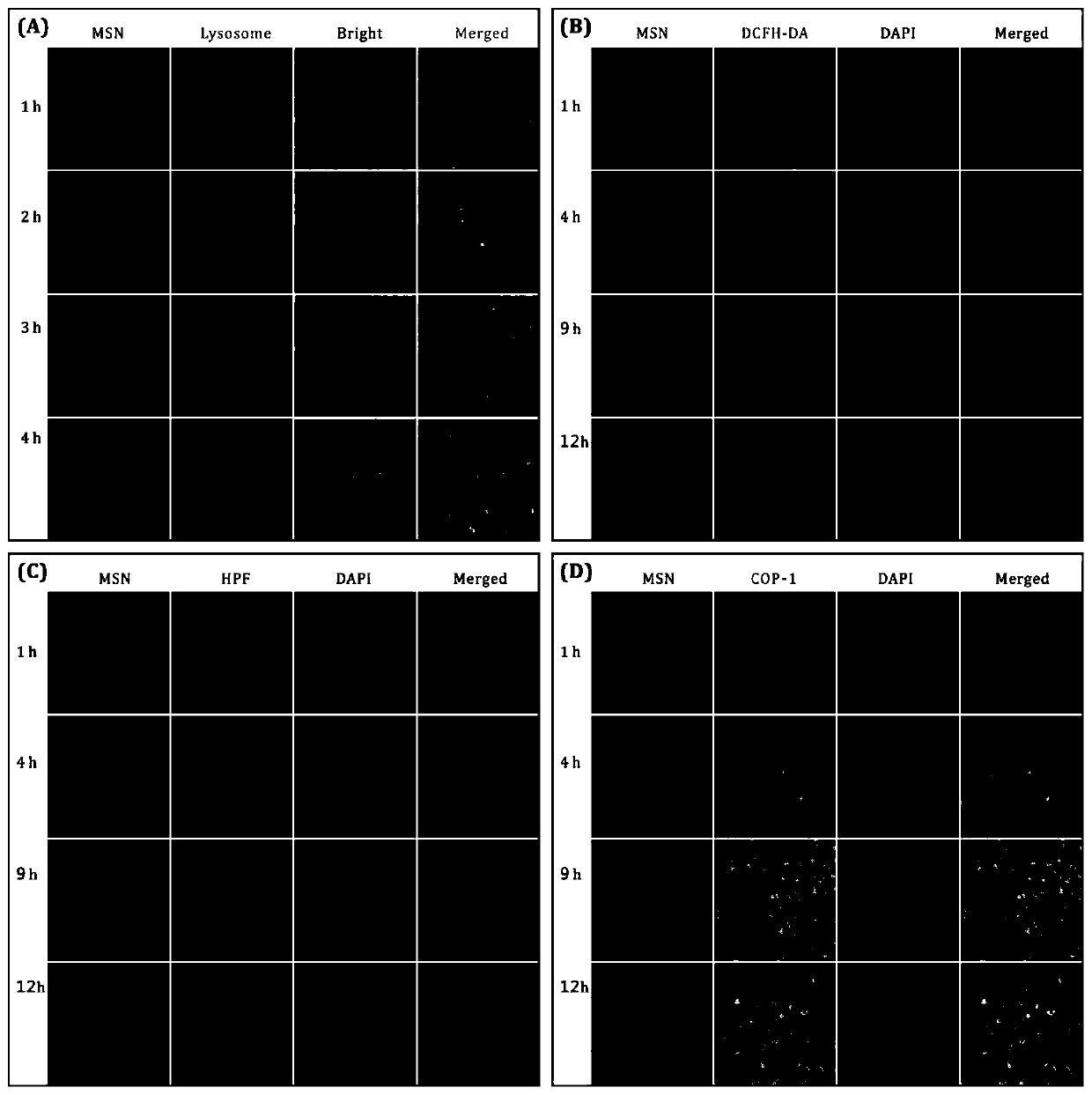
Tumor-targeted nanomedicine and application and preparation method
A nano-drug and tumor-targeting technology, applied in the field of tumor-targeting nano-drugs and their application and preparation, can solve the problems of complex drug synthesis process, lack of release specificity, exogenous stimulation, etc., achieving high synthesis efficiency and reducing production The effect of low cost and raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of described nano medicine comprises the following steps:
[0030] Step 1, using template method to synthesize MSN nanoparticles.
[0031] 2g of CTAC and 0.02g of TEAH were sequentially dissolved in 20ml of deionized water, stirred to fully dissolve. After stirring at room temperature for 10 min, it was transferred to a water bath at 80 °C and stabilized for 30 min. Then, a total of 1.5 ml of tetraethyl orthosilicate was added dropwise, and heating was continued for 1 h under the stirring condition of 400 r / min. The product was collected by centrifugation and washed twice with ethanol to remove additional products of the reaction. The resulting product was then ultrasonically dispersed in 10 mL of deionized water.
[0032] Described Tetraethyl orthosilicate (TEOS), triethanolamine (TEAH), cetyltrimethylammonium chloride (CTAC) are chemical raw materials commonly used in the preparation of this field, all can be ordered from the reagent net.
...
Embodiment 1
[0058] (1) Preparation of MSN nanoparticles.
[0059] 2g of CTAC and 0.02g of TEAH were sequentially dissolved in 20ml of deionized water, stirred to fully dissolve. After stirring at room temperature for 10 min, it was transferred to a water bath at 80 °C and stabilized for 30 min. Then a total of 1.5ml of tetraethyl orthosilicate was added dropwise, and the heating was continued for 1h under the stirring condition of 400r / min. The product was collected by centrifugation and washed twice with ethanol to remove the additional product of the reaction. The resulting product was then ultrasonically dispersed in 10 mL of deionized water to obtain a MSN solution.
[0060] (2) Preparation of MSNs deposited with manganese dioxide in mesoporous channels (MnO 2 @MSN).
[0061] After the above solution was stirred at 40°C for 15min, 10mL KMnO4 aqueous solution (2.5-25mM) was slowly added dropwise under magnetic stirring, and reacted in a water bath at 40°C in the dark for 4h. The p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Mesopore diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com