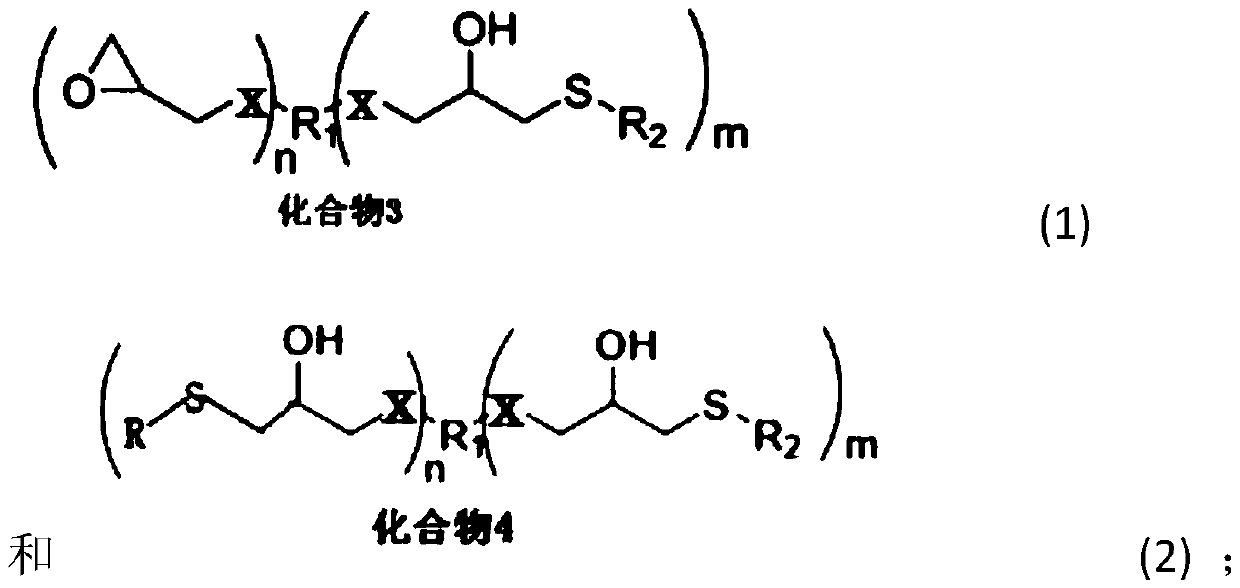
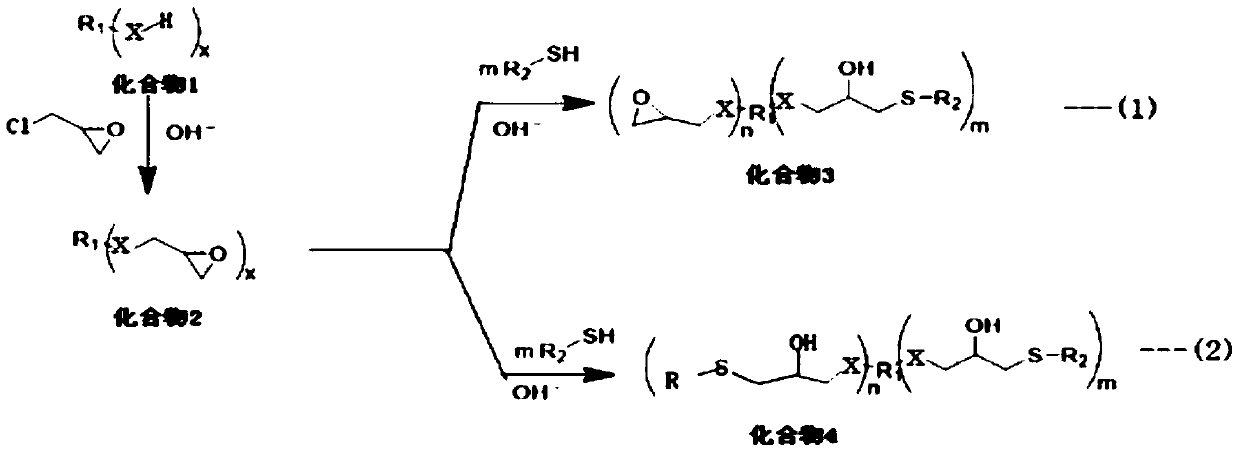
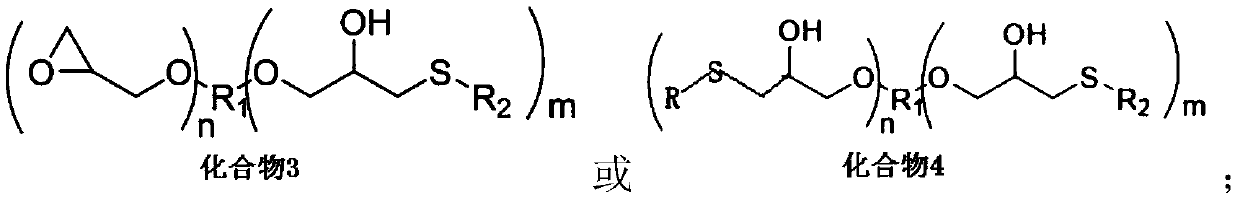
Novel chelate-polymer antioxidative stabilizer, preparation method therefor and application of novel chelate-polymer antioxidative stabilizer
An anti-oxidative stabilizer and anti-oxidation technology, applied in chemical instruments and methods, thioether preparation, organic chemistry, etc., can solve problems such as increasing environmental pollution, affecting the use function and life of polymer materials, and threats to life safety. The effect of good matching, maintaining mechanical properties, and improving protection performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The structural formula of target product 1
[0023]
[0024] Synthesis of target product 1:
[0025] Step (1): preparation of bisphenol A diglycidyl ether (compound 2);
[0026] Add 1mol (ie 228g) of bisphenol A and 2.8mol (ie 260g) of epichlorohydrin into a 2-liter three-necked flask, add a reflux tube, protect with N2, heat and stir at 70°C, when the internal temperature reaches 65°C, divide into 4 batches Slowly add 2.05mol (ie 82g) sodium hydroxide, keep warm until the reaction of bisphenol A is completed, add 1L MTBE after cooling down to 50°C, filter the insoluble matter, and recover MTBE and epichlorohydrin from the filtrate under reduced pressure to obtain colorless The oily substance, 306 g of bisphenol A diglycidyl ether, was directly used in the next step without further purification.
[0027] Step (2) synthesis of target product 1;
[0028] When compound 2 is bisphenol A bisglyceryl ether, the thiol is n-dodecanethiol.
[0029] Add 0.20 mol (ie 38.2 g...
Embodiment 2
[0031] Chemical synthesis target product 2 Example synthesis steps:
[0032] Target Product 2 Structural Formula
[0033]
[0034] Target product 2 synthesis steps:
[0035] When compound 2 is bisphenol F bisglyceryl ether, the thiol is n-dodecanethiol, if m=2.0, n=0. The reaction steps are: add 313 grams of bisphenol F bisglyceryl ether, 1200 ml of anhydrous dichloromethane or dichloroethane or acetone or acetonitrile into a 2.5L reaction bottle, stir until dissolved, add n-dodecanethiol 405 gram, nitrogen protection, ice bath for half an hour, slowly add 5 grams of sodium hydroxide, naturally warm up to room temperature and then insulate and stir for 20 hours, detect that the mercaptan reaction is complete, reclaim dichloromethane under reduced pressure, freeze to obtain 715 grams of white solid, and produce The rate is 99.6%.
[0036] (ESI / MS+: 493.71, 515, 739.6, 827.5, 1029.48; 1 H-NMR / 400MHZ, CDCl 3 :δ7.11–7.03(m,1H),6.83(ddd,J=8.6,4.3,2.2Hz,1H),4.05–3.89(m,2H),2...
Embodiment 3
[0038] Chemical synthesis target product 3 example synthesis steps:
[0039] Target Product 3 Structural Formula
[0040]
[0041] Target product 3 synthesis steps:
[0042] When compound 2 is triglycidyl isocyanurate (TGIC), and the thiol is n-dodecanethiol, add 29.7 grams of TGIC, 120 ml of anhydrous ethyl acetate or anhydrous dichloromethane or anhydrous dichloromethane to a 250ml reaction bottle. Ethyl chloride, stirred until dissolved, added 60.7 grams of n-dodecanethiol, under nitrogen protection, slowly added 0.5 grams of sodium hydroxide, stirred at room temperature for 16 hours, detected that the mercaptan reaction was complete, filtered to obtain 90 grams of white solid, and the yield 99.6%.
[0043] ESI / MS+: 904.52, 701.98; 1 H-NMR / 400MHZ, CDCl 3:δ6.54(s,1H),4.18–3.80(m,1H),2.79–2.37(m,1H),1.98(s,1H),1.51(p,J=7.4Hz,1H),1.19(s ,6H),0.81(t,J=6.7Hz,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com