Method for measuring entacapone tablet dissolution rate through utilization of high performance liquid chromatography
A technology of high performance liquid chromatography and entacapone, which is applied in the field of high performance liquid chromatography to determine the dissolution rate of entacapone tablets, can solve the problems of limited accuracy, cumbersome operation and the like, and achieve the effect of efficient evaluation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Chromatographic condition selection:
[0024] Diluent: Phosphate buffer solution with pH=5.5; Buffer: 2.1g / L sodium dihydrogen phosphate solution, adjust pH to 2.1 with phosphoric acid; Mobile phase: methanol, tetrahydrofuran, buffer solution=60:2:38; Wavelength: UV 300nm; Chromatographic column: 4.6mm*250mm; 5μm phenyl column; Column temperature: 30°C; Flow rate: 1.0ml / min; Injection volume: 10μl.
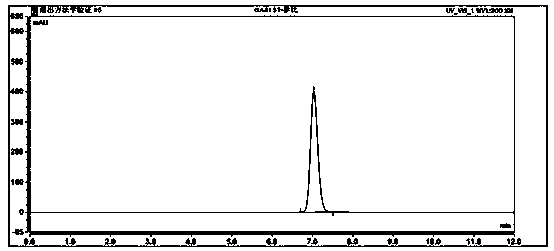
[0025] Specificity experiment of the present invention: enter blank solvent, blank auxiliary material, reference substance solution, and test product solution respectively to investigate the specificity of the method, and confirm that the blank solvent and auxiliary materials of this verification method do not interfere with the determination of entacapone.
[0026] The solution was prepared as follows:
[0027] Blank solvent: phosphate solution with pH=5.5: 6.8g / l potassium dihydrogen phosphate solution, adjust the pH to 5.5 with 0.5M sodium hydroxide.
[0028] Blank exc...
Embodiment 2
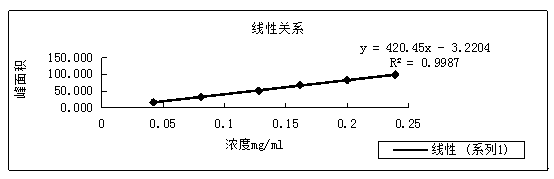
[0034] Linear correlation experiment of the inventive method
[0035] Take about 20 mg of entacapone reference substance, accurately weigh it, put it in a 25ml measuring bottle, add 20% methanol, and ultrasonically dissolve it, then add a certain amount of medium to dilute and set the volume to the mark, as a stock solution.
[0036] Accurately measure 1ml, 2ml, 3ml, and 5ml of the above-mentioned stock solution respectively, put them in a 20ml measuring bottle, add dissolution medium to dissolve and dilute to the scale, and prepare solutions with concentrations of 20%, 40%, 60%, and 100%; Put 2ml and 3ml of the stock solution into 10ml measuring bottles respectively, add dissolution medium to dissolve and dilute to the mark, and prepare 80% and 120% concentration solutions.
[0037] Analyze according to the detection method of the present invention, the injection volume is 10 μ l, take the concentration of entacapone as the abscissa, and the peak area as the ordinate to make ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com