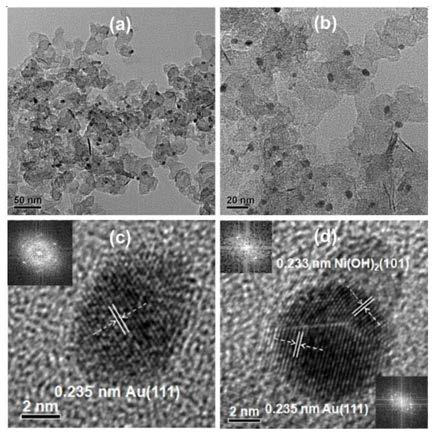
Bimetal catalyst for preparing m-nitroaniline through hydrogenation of m-dinitrobenzene
A technology of bimetallic catalyst and m-dinitrobenzene, which is applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, amino compound preparation, etc., can solve the problem of high cost of electrode materials and electrolysis equipment, It is unfavorable for industrialized production, a large amount of iron sludge and other problems, and the method is green and efficient, the method is simple, and the effect of improving catalytic performance is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Preparation of Ni / C, Au / C, AuNi / C catalysts
[0023] The preparation method of Ni / C: weigh 0.9000-1.8000g of NiCl 2 ·6H 2 O in a 250ml Erlenmeyer flask, then add 60-100ml of ethylene glycol and 10-40ml of deionized water, and ultrasonically disperse for 10-40 minutes; add the prepared 5-10ml of NaOH solution (or Na 2 CO 3 ), magnetically stirred for 10-20 minutes; weighed 1.2500g of carbon carrier and added it to the Erlenmeyer flask, aged for 4-10h; pipetted 10-40ml of hydrazine hydrate (or sodium borohydride ethylene glycol solution) into the above solution, and magnetically stirred 10 minutes; transfer the solution in the Erlenmeyer flask to the lining of the autoclave, and magnetically stir for 15-24 hours; filter and wash, wash with ethanol and deionized water several times, vacuum dry, grind, and weigh to obtain Ni / C ;
[0024] The preparation method of Au / C: pipette a certain amount of HAuCl 4 Put the solution in a beaker, add 0.3g of carbon carrier, hea...
Embodiment 3
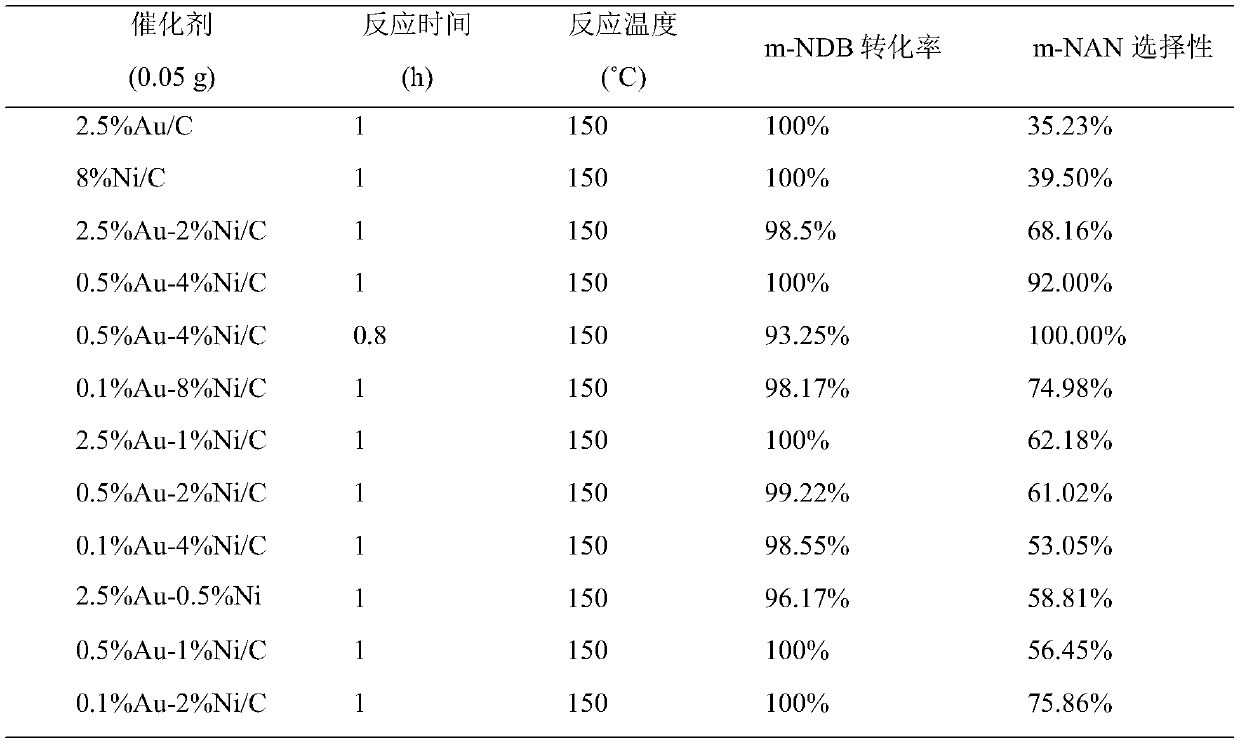
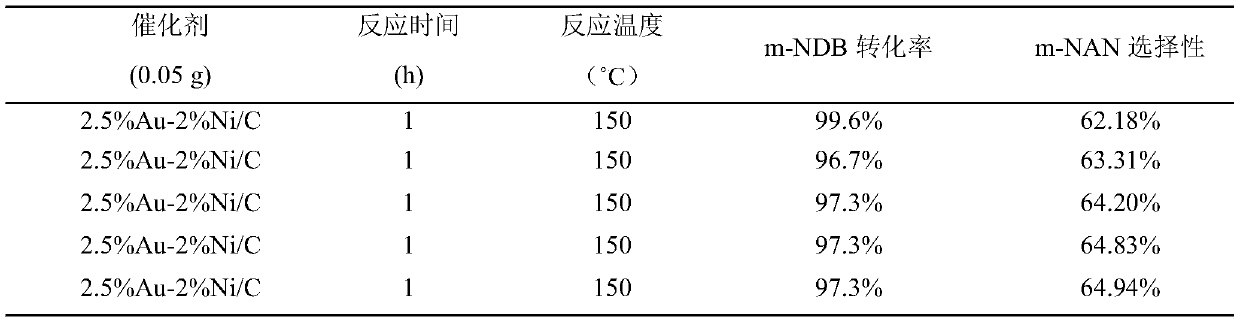
[0031] The 2.5%Au-2%Ni / C catalyst prepared in Example 1 was used to carry out the experiment of preparing m-nitroaniline by selective hydrogenation of m-dinitrobenzene. Weigh 0.05g of 2.5%Au-2%Ni / C catalyst in an autoclave, pipette 10ml of m-dinitrobenzyl alcohol solution, and stir at a reaction temperature of 150°C, a hydrogen pressure of 3MPa, and a rotating speed of 870r / min After reacting under the same conditions for 1 hour, the separated clear liquid was analyzed by gas chromatography. The conversion rate of m-dinitrobenzene was 100%, and the selectivity of m-nitroaniline was 68.16%. The reaction results are shown in attached table 1.
Embodiment 4
[0033] The 0.5%Au-4%Ni / C catalyst prepared in Example 1 was used to carry out the experiment of preparing m-nitroaniline by selective hydrogenation of m-dinitrobenzene. Weigh 0.05g of 0.5%Au-4%Ni / C catalyst in an autoclave, pipette 10ml of m-dinitrobenzyl alcohol solution, and stir at a reaction temperature of 150°C, a hydrogen pressure of 3MPa, and a rotating speed of 870r / min After reacting under the conditions for 1 h, the separated clear liquid was analyzed by gas chromatography. The conversion rate of m-dinitrobenzene was 100%, and the selectivity of m-nitroaniline was 92.00%. The reaction results are shown in attached table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com