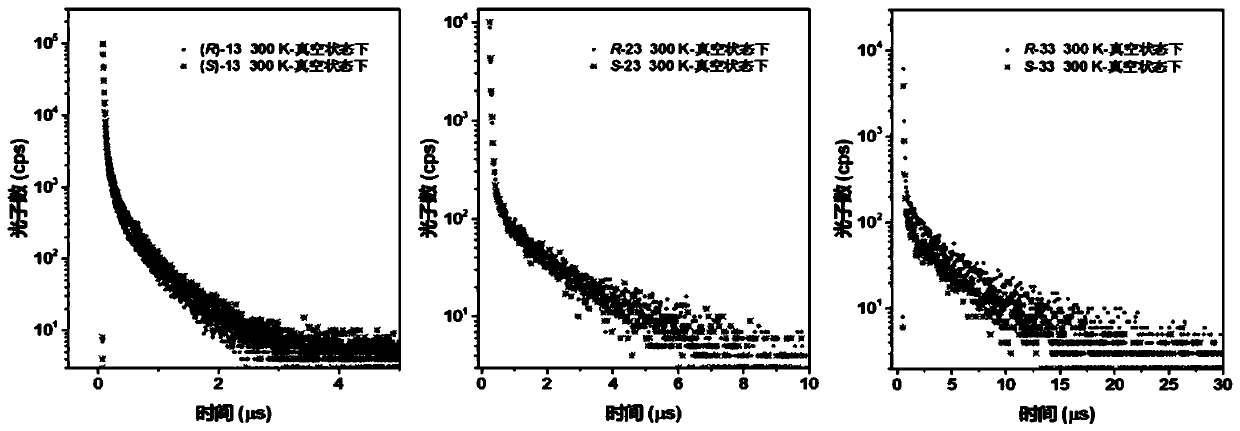
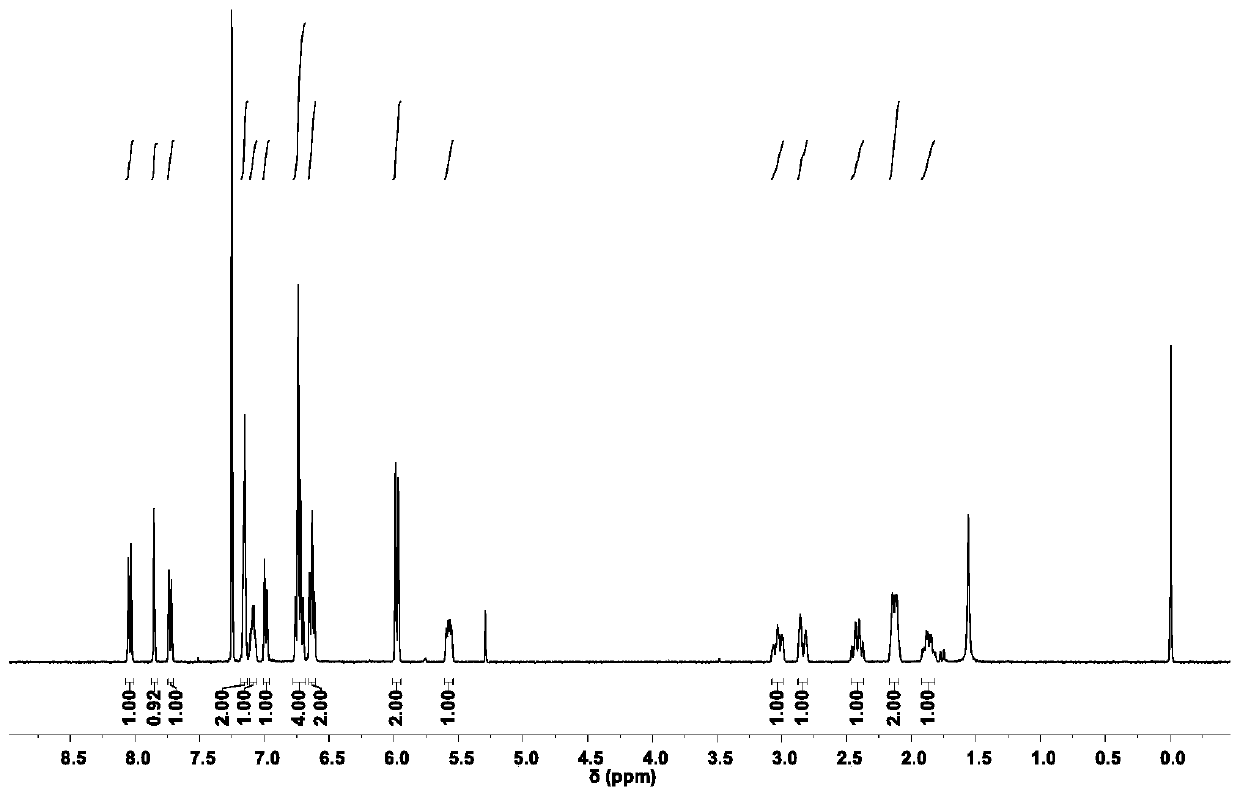
Aggregation-induced emission luminescent material with circular polarization luminescent and thermally activated delayed fluorescence emission performance, and preparation method and applications thereof
A technology of aggregation-induced luminescence and thermal activation delay, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc. , the effect of high yield and high luminous brightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Synthesis of target compound (I):
[0080] 1) Synthesis of compound 1:
[0081] Under argon protection, 4-bromophthalic anhydride (1.50g, 6.64mmol) and (R)-1,2,3,4-tetrahydro-1-naphthol (0.98g, 6.64mmol) were added into a three-neck flask, dissolved with 15 mL of DMF, and stirred at 150°C for 24 hours. After the reaction solution was cooled to room temperature, pour it into 150mL water to precipitate the solid, filter it with suction, and use the mixed solution of dichloromethane and petroleum ether with a volume ratio of 1:2 as the mobile phase to separate and purify the crude product by silica gel column chromatography and dry it in vacuo Obtain white powder 1.84g (compound 1, R-ImNBr) afterward, about 78% of productive rate, reaction formula is as follows:
[0082]
[0083] 2) Synthesis of target compound (I):
[0084] Compound 1 (1.00g, 2.82mmol), phenoxazine compound (0.70g, 3.52mmol), anhydrous potassium phosphate (0.75g, 3.52mmol), 2-dicyclohexylphosphine-2...
Embodiment 2
[0087] Synthesis of target compound (I):
[0088] 1) Synthesis of compound 1:
[0089] Under argon protection, 4-bromophthalic anhydride (1.50g, 6.64mmol) and (S)-1,2,3,4-tetrahydro-1-naphthol (0.98g, 6.64mmol) were added into a three-neck flask, dissolved with 15 mL of DMF, and stirred at 150°C for 24 hours. After the reaction liquid is cooled to room temperature, pour it into 150mL water to precipitate the solid, filter it with suction, and use the mixed solution of dichloromethane and petroleum ether with a volume ratio of 1:2 as the mobile phase to separate and purify the crude product by silica gel column chromatography and dry it in vacuo Obtain white powder 1.89g (compound 1, S-ImNBr) afterward, about 80% of productive rate, reaction formula is as follows:
[0090]
[0091] 2) Synthesis of target compound (I):
[0092] Compound 1 (1.00g, 2.82mmol), phenoxazine compound (0.70g, 3.52mmol), anhydrous potassium phosphate (0.75g, 3.52mmol), Ruphos (0.26g, 0.56mmol) wer...
Embodiment 3
[0095] Synthesis of target compound (II):
[0096] 1) Synthesis of compound 1:
[0097] The synthetic method of compound 1 is the same as the synthetic method of compound 1 in embodiment 1;
[0098] 2) Synthesis of target compound (II):
[0099] Add compound 1 (1.00g, 2.82mmol), dimethylacridine compound (0.74g, 3.52mmol), anhydrous potassium phosphate (0.75g, 3.52mmol), Ruphos (0.26g, 0.56mmol) into the three-necked flask , dissolved in 30 mL of toluene, stirred and bubbled with argon for 30 minutes, then added Pd 2 (dba) 3 (80mg, 0.14mmol), stirred and reacted at 120°C for 24 hours under the protection of argon. After the reaction solution was cooled to room temperature, it was filtered with suction, and the solvent was distilled off by a rotary evaporator under reduced pressure. The obtained crude product was separated and purified by silica gel column chromatography using a mixed solution of dichloromethane and petroleum ether with a volume ratio of 1:2 as the mobile p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com