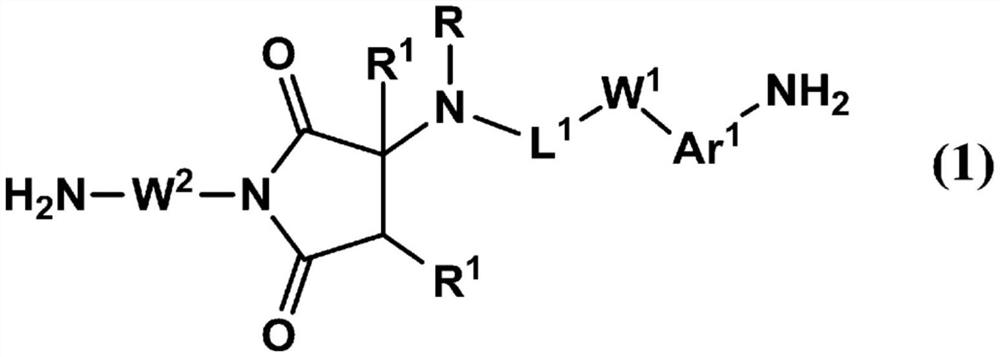
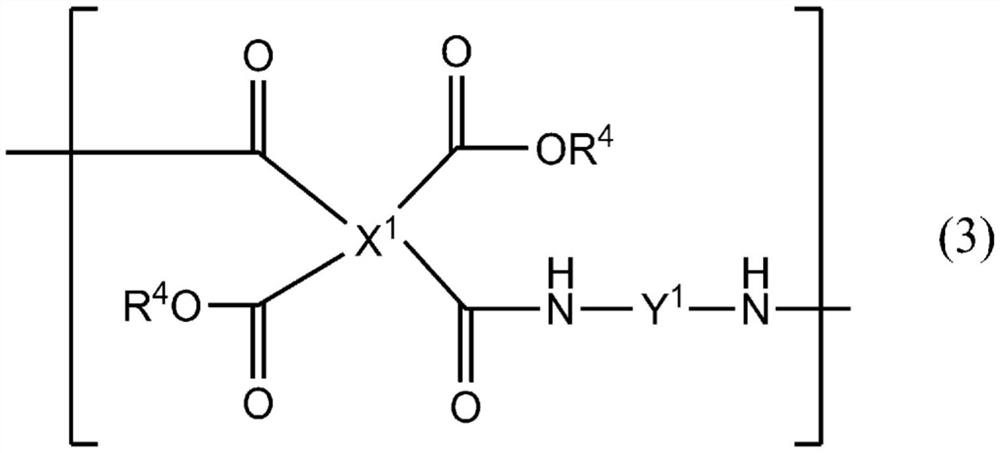
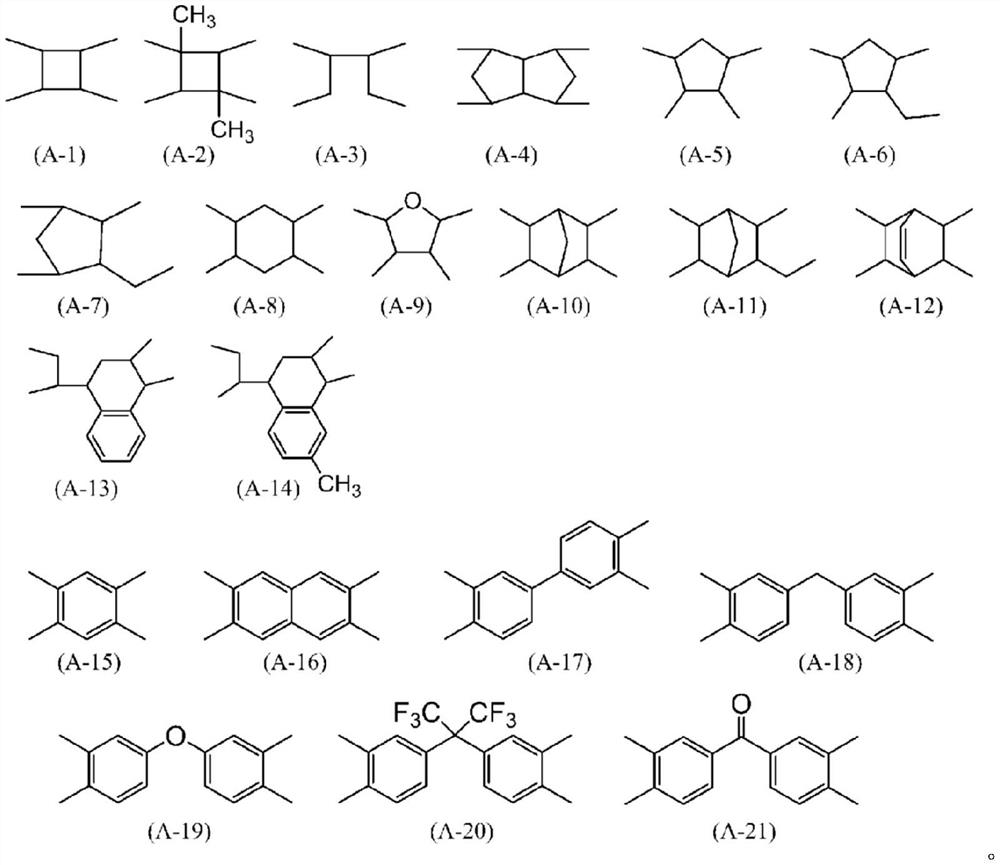
Liquid crystal alignment agent, liquid crystal alignment film and liquid crystal display element
A technology of liquid crystal alignment agent and structural unit, applied in liquid crystal materials, chemical instruments and methods, instruments, etc., can solve problems such as brightness reduction, gray scale display obstacles, long-term reliability reduction, etc., and achieve the effect of promoting movement and promoting relaxation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] Synthesis of (DA-1-1)
[0203]
[0204]After adding 15.00 g (68.8 mmol) of N-(4-nitrophenyl)maleimide and 300 g of tetrahydrofuran (hereinafter referred to as THF) to the flask, it was cooled with ice. To the mixture was added 10.84 g (72.2 mmol) of 4-(2-methylaminoethyl)aniline. Then, after gradually returning to room temperature, it stirred at room temperature for 3 hours. After confirming the completion of the reaction, THF was distilled off under reduced pressure. To the obtained residue was added n-hexane, followed by stirring. The obtained precipitate was filtered. When the obtained crystal was dried at 50°C, 21.7 g of the target nitro body intermediate (DA-1-1-1) was obtained (yield: 84%).
[0205] 1H-NMR (D6-DMSO, δppm): 8.36 (d, 2H), 7.61 (d, 2H), 6.88 (d, 2H), 6.49 (d, 2H), 4.84 (brs, 2H), 4.20-4.25 ( m, 1H), 2.91-3.00 (m, 1H), 2.65-2.83 (m, 3H), 2.54-2.61 (m, 2H), 2.37 (s, 3H)
[0206] Into the nitrogen-substituted flask, 10 g (27.1 mmol) of (DA1-1-1...
Embodiment 2
[0209] Synthesis of (DA-1-2)
[0210]
[0211] After adding 10.00 g (45.8 mmol) of N-(4-nitrophenyl)maleimide and 200 g of THF to the flask, it was cooled with ice. To the mixture was added dropwise 22.4 g (50.0 mmol) of a 7%-methylamine-THF solution. Then, it stirred under ice cooling for 3 hours. After confirming the completion of the reaction, when THF was distilled off under reduced pressure, the target intermediate (DA-1-2-1) was quantitatively obtained as white crystals.
[0212] 1H-NMR (D6-DMSO, δppm): 8.38 (d, 2H), 7.62 (d, 2H), 3.81-3.87 (m, 1H), 3.03-3.14 (m, 1H), 2.56-2.66 (m, 1H) ), 2.66(brs, 1H), 2.42(s, 3H)
[0213] After adding 11.4 g (45.7 mmol) of (DA-1-2-1), 200 g of THF, and 5.10 g (50.4 mmol) of triethylamine to the flask, it was cooled with ice. To the mixture was added 8.08 g (43.5 mol) of 4-nitrobenzoyl chloride, followed by stirring under ice-cooling for 3 hours. After confirming the completion of the reaction, the precipitate was filtered. Whe...
Embodiment 3
[0218] Synthesis of (DA-1-3)
[0219]
[0220] After adding 15.66 g (71.8 mmol) of N-(4-nitrophenyl) maleimide and 300 g of THF to the flask, it was cooled with ice. To the mixture was added dropwise 35.0 g (78.9 mmol) of a 7%-methylamine-THF solution. Then, it stirred under ice cooling for 3 hours. After confirming the completion of the reaction, when THF was distilled off under reduced pressure, the target intermediate (DA-1-2-1) was quantitatively obtained as white crystals.
[0221] After adding 17.8 g (71.4 mmol) of (DA-1-2-1) obtained above, 300 g of THF, and 7.99 g (79.0 mmol) of triethylamine to the flask, it was cooled with ice. To the mixture was added 15.1 g (68.1 mol) of 4-nitrobenzenesulfonyl chloride, followed by stirring at 40°C for 14 hours. After confirming the completion of the reaction, the precipitate was filtered. When the obtained filtrate was distilled off under reduced pressure, the crude product (DA-1-3-1) was obtained. To the obtained crude pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com