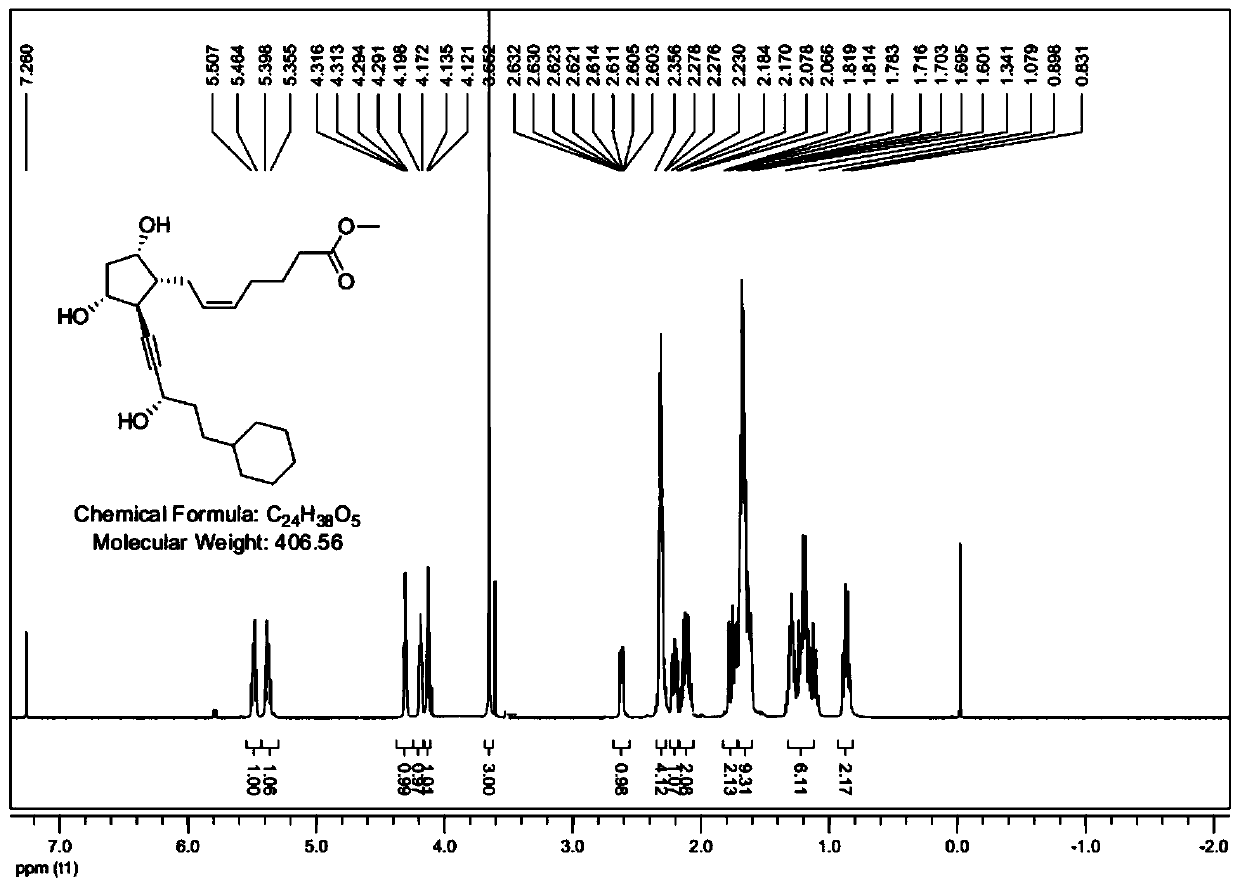
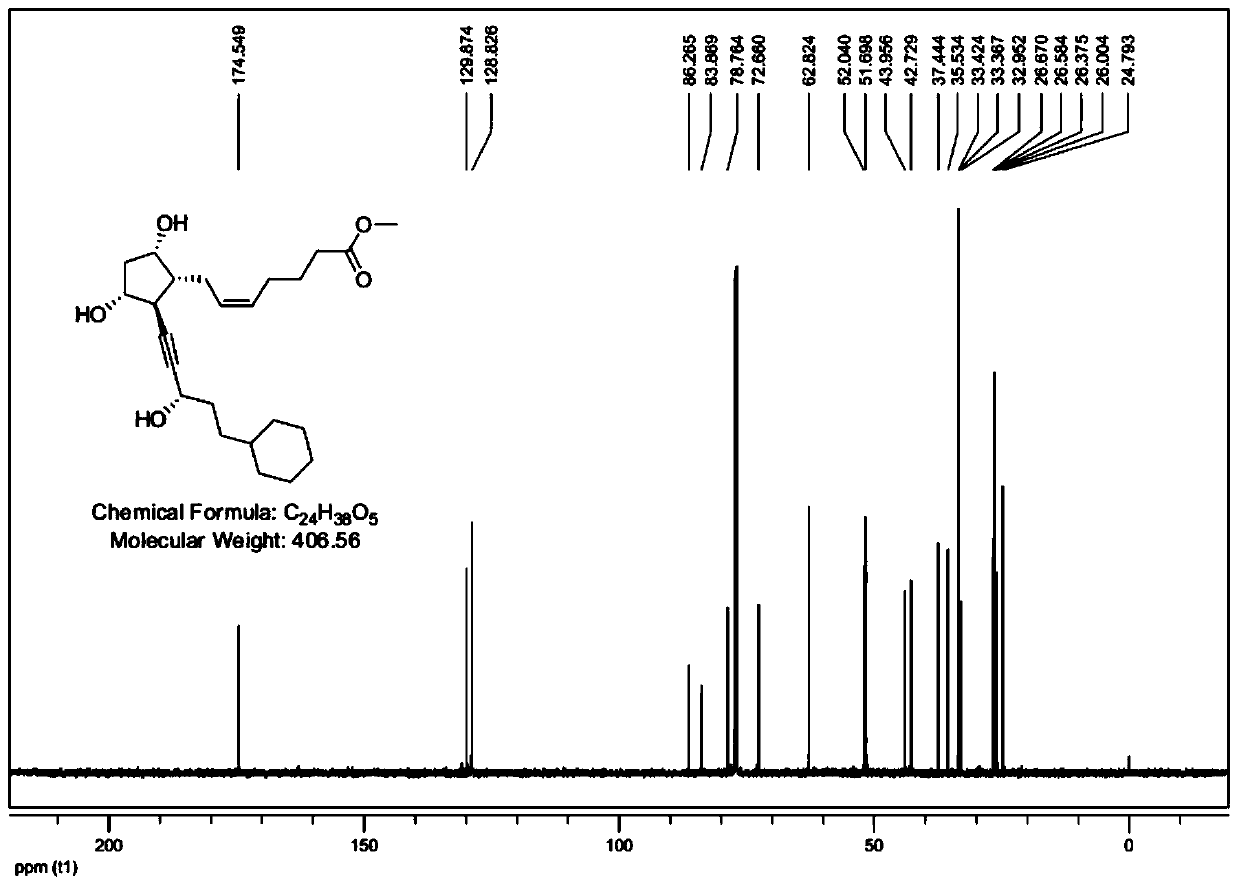
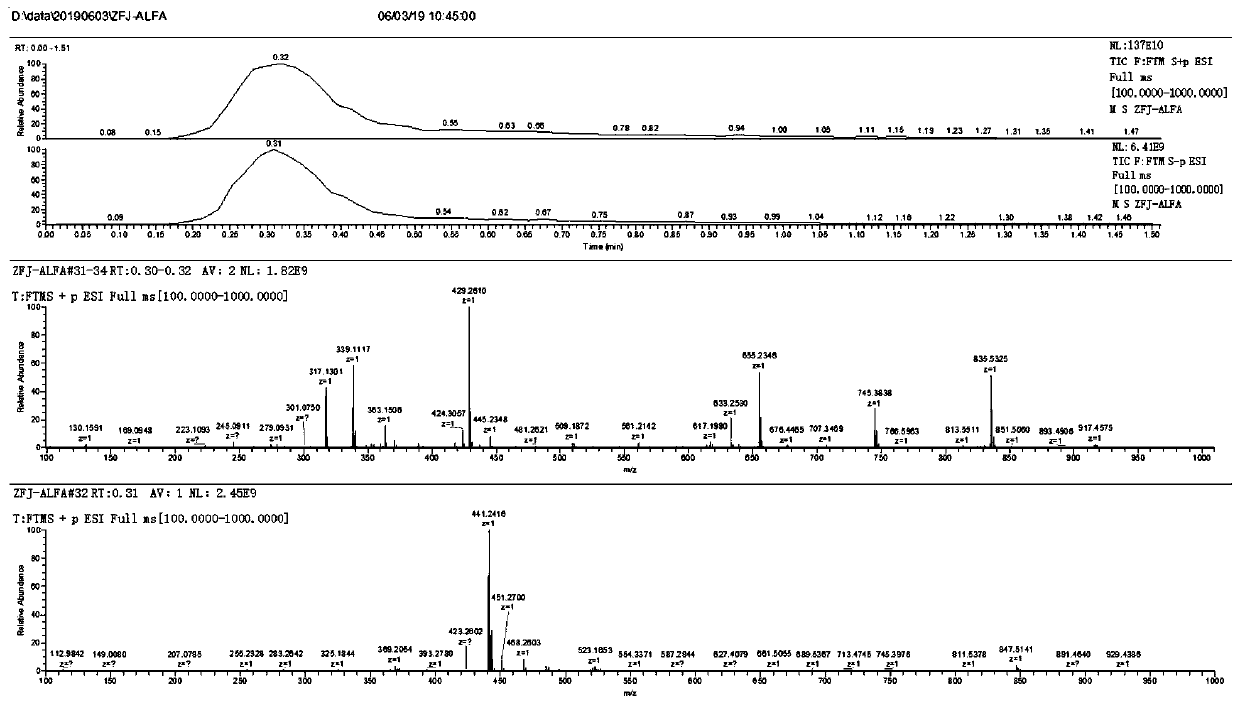
Synthetic method of alfaprostol
A technology of afaprostol and a synthesis method, which is applied in the field of pharmaceutical preparation, can solve the problems of being unsuitable for industrial scale-up production, having severe requirements for anhydrous and oxygen-free operation, and being unsuitable for industrial scale-up production, and achieving easy control of isomer impurities, Simple post-processing and easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0043] In order to make the purpose, technical solutions and advantages of the embodiments of the present invention more clear, the following will be clearly and completely described in conjunction with the technical solutions in the embodiments of the present invention. Obviously, the described embodiments are part of the embodiments of the present invention, rather than Full examples. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
[0044] The invention provides a kind of synthetic method of alfaprostol, comprising the following steps:
[0045] Step a, adding L-benzoyl coli lactone and an oxidizing agent into a solvent, performing primary alcohol oxidation reaction, and then quenching, extracting, washing, drying, filtering, and concentrating to obtain aldehyde-containing coli lactone, namely Compound a2;
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com