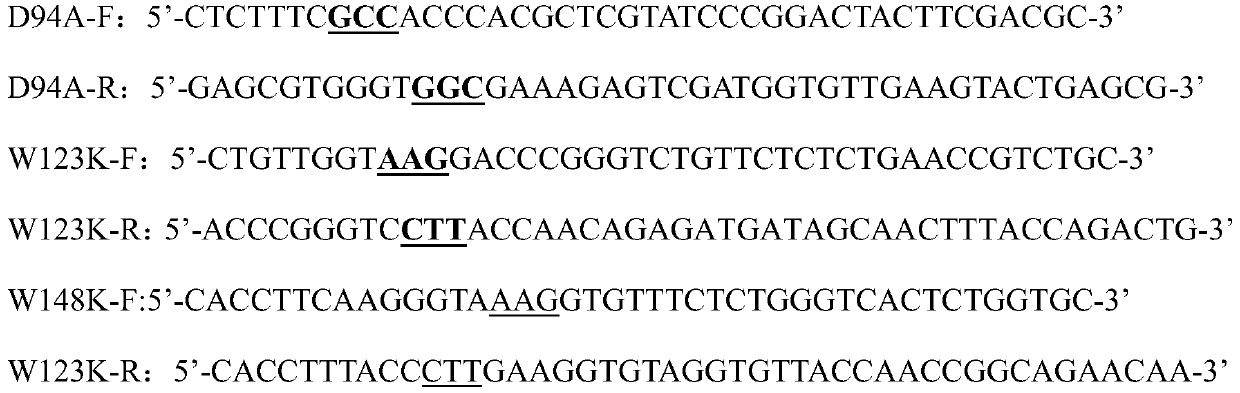Meso-diaminopimelic acid dehydrogenase mutants with improved catalytic efficiency
A technology of diaminopimelate dehydrogenase and mesization, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Construction of meso-diaminopimelate dehydrogenase mutant
[0028] With the pET-28a recombinant plasmid containing the meso-diaminopimelate dehydrogenase gene (shown in SEQ ID NO.1) derived from Ureibacillus thermosphaericus (Ureibacillus thermosphaericus) method build) as a template.
[0029] Use the oligonucleotide fragment containing the mutation point as the homology arm to design upstream and downstream primers. The specific primers are as follows (the bold and underlined are the mutation sites):
[0030]
[0031] The mutant plasmids were constructed by inverse PCR of the whole plasmid.
[0032] PCR amplification system: template 1 μL, upstream and downstream primers 0.5 μL, 2×Phanta Max Master Mix polymerase 10 μL, sterilized ddH 2 O 8 μL, total reaction system 20 μL. PCR reaction conditions: 95°C pre-denaturation, 5min; 95°C denaturation, 30s, 58°C annealing, 30s, 72°C extension, 2min, 30 cycles; 72°C full extension, 10min.
[0033] The PCR prod...
Embodiment 2
[0035] Example 2: Induced expression of meso-diaminopimelate dehydrogenase mutants
[0036] Inoculate the engineered meso-diaminopimelate dehydrogenase mutant engineered bacteria constructed in Example 1 into LB liquid medium containing 50 μg / mL kanamycin, cultivate overnight at 37°C and 180 r / min, and then transfer In 50mL of LB medium. The inoculum size is 1%, the culture temperature is 37°C, and the rotation speed is 180r / min. Grow to OD 600After reaching 0.6-0.9, add IPTG with a final concentration of 0.5mM for induction. The induction temperature is lowered to 16°C. After 14 hours of induction, the bacteria are collected by centrifugation at 8000rpm at 4°C for 10 minutes, and stored in a -70°C refrigerator for later use.
Embodiment 3
[0037] Example 3: Separation and purification of meso-diaminopimelate dehydrogenase mutant
[0038] Take the wet bacterial cells collected in Example 2, wash twice with 10mL of 50mM PBS buffer solution of pH 7.5, resuspend in 10mL of 50mM PBS buffer solution of pH 7.5, oscillate and shake well, and break under ultrasonic waves for 1s , stop for 3s, the total duration is 15min. The cell lysate was centrifuged at 12,000 rpm for 20 min to remove cell debris, and the supernatant, namely the crude enzyme solution, was collected and filtered with a 0.22 μm filter membrane for subsequent separation and purification of the enzyme.
[0039] The purification column is a Ni-NTA column with a packing volume of 5mL, and the loading equilibration buffer M 0 (20mM Tris, 500mMNaCl, pH 7.4) equilibrate the Ni-NTA column, load the crude enzyme solution at a rate of 0.5mL / min, and use the loading equilibration buffer M 0 Elution to remove unadsorbed protein, and finally with elution buffer M ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com