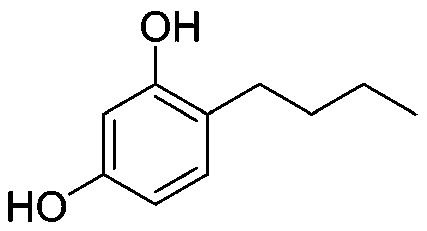
Method for synthesizing 4-n-butylresorcinol by microchannel reaction
A technology of n-butyl resorcinol and n-butyryl resorcinol, which is applied in the field of cosmetic intermediate synthesis, can solve problems such as hidden safety hazards and increased difficulty, save equipment space and reduce waste acid and residue , the effect of improving the reaction yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
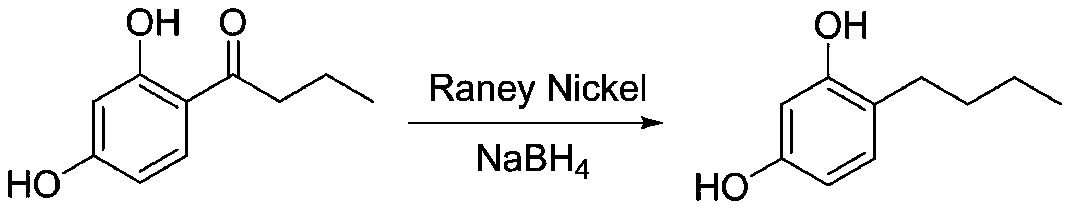
[0037] Weigh 100g of 4-n-butyryl resorcinol and dissolve it in toluene, cool it in ice to 0°C, then slowly add 32g (1.5eq) sodium borohydride in batches, and react at room temperature for 12 hours after the addition; then pour it into the pre- Heat module 1, preheat to 80°C; mix 67g of Raney nickel and isopropanol into preheat block 2, and preheat to 80°C. Then add the above two preheated materials into the reaction module, and the residence time is controlled at 200s. Collect the reaction solution flowing out, pour into ice water to quench, filter off the solid, evaporate most of the toluene, add 330mL of dichloromethane for extraction, evaporate the dichloromethane to dryness, and obtain 82g of white solid 4-n-butylresorcinol, Yield 89%, HPLC purity 99.3%.
Embodiment 2
[0039] Weigh 100g of 4-n-butyryl resorcinol and dissolve it in isopropanol, put it in an ice bath to 0°C, then slowly add 32g (1.5eq) of sodium borohydride in batches, and react at room temperature for 12 hours after the addition; Inject into preheating module 1 and preheat to 80°C; mix 67g of Raney nickel and isopropanol into preheating module 2 and preheat to 80°C. Then add the above two preheated materials into the reaction module, and the residence time is controlled at 200s. Collect the reaction solution flowing out, pour it into ice water to quench, filter off the solid, distill off the excess isopropanol, add 350 mL of dichloromethane for extraction, evaporate the dichloromethane to dryness, and obtain 89 g of white solid 4-n-butylresorcinol , yield 96%, HPLC purity 99.6%.
Embodiment 3
[0041] Weigh 100g of 4-n-butyryl resorcinol and dissolve it in isopropanol, put it in an ice bath to 0°C, then slowly add 21g (1eq) of sodium borohydride in batches, and react at room temperature for 12 hours after the addition; then pour it into Preheat module 1, preheat to 80°C; mix 67g of Raney nickel and isopropanol into preheat module 2, and preheat to 80°C. Then add the above two preheated materials into the reaction module, and the residence time is controlled at 200s. Collect the reaction solution flowing out, pour it into ice water to quench, filter off the solid, evaporate the excess isopropanol, add 350 mL of dichloromethane for extraction, evaporate the dichloromethane to dryness, and obtain 84 g of white solid 4-n-butylresorcinol , yield 91%, HPLC purity 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com