A kind of solid-state blue fluorescent carbon quantum dot with crystallization-induced luminescence enhancement and preparation method thereof
A technology of crystallization-induced luminescence and carbon quantum dots, which is applied in the preparation of carbon quantum dots, carbon quantum dot materials, and carbon quantum dots, can solve the problems of weakening and disappearing fluorescence intensity, and achieve high fluorescence quantum yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 1.6214 g of phloroglucinol dihydrate and 0.6006 g of urea into 10 mL of distilled water, and sonicate for 3 min to obtain a uniformly dispersed reaction solution.
[0037] The reaction solution was placed in a 100mL glass beaker and reacted in a 144W microwave oven for 15min. After the reaction was completed, it was cooled to room temperature, and the reaction solution was taken out, and placed in a -80°C refrigerator for 30 minutes to freeze, and a crystalline solid product was precipitated.
[0038] After the solid product is placed on filter paper to absorb excess water, it is placed in a vacuum oven to dry at room temperature to obtain a white crystalline blue fluorescent carbon quantum dot solid powder.
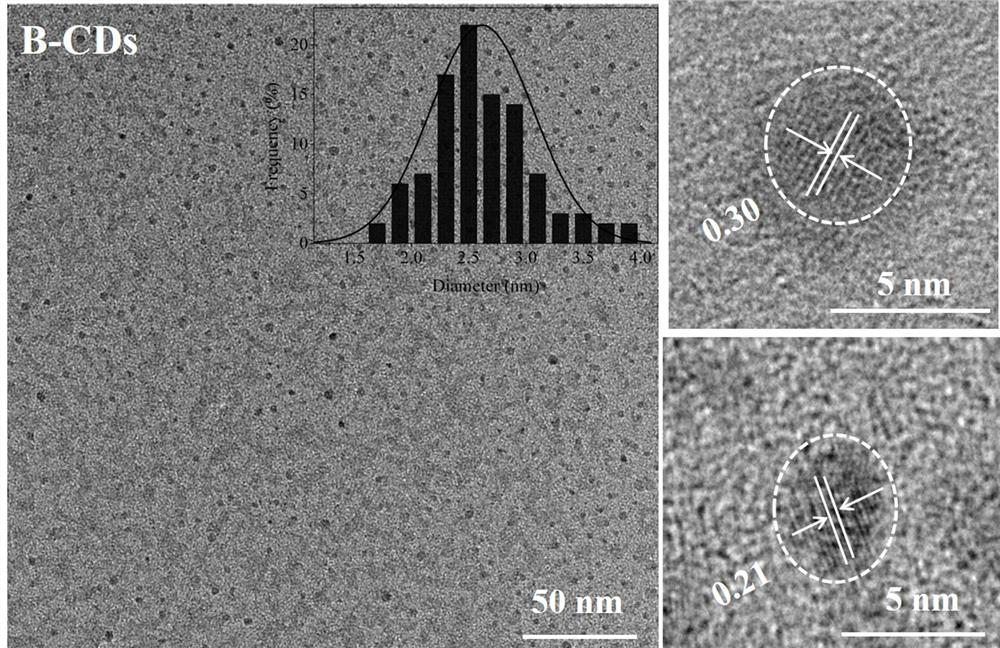
[0039] figure 1 It is the TEM photo of the prepared blue fluorescent carbon quantum dots. It can be seen from the figure that the blue fluorescent carbon quantum dots are uniformly dispersed, the particle size distribution is 1.75-4.00nm, the average particl...
Embodiment 2
[0050] Add 1.6214 g of phloroglucinol dihydrate and 9.6096 g of urea into 10 mL of distilled water, and sonicate for 3 min to obtain a uniformly dispersed reaction solution.
[0051] The reaction solution was placed in a 100mL glass beaker and reacted in a 130W microwave oven for 15min. After the reaction was completed, it was cooled to room temperature, and the reaction solution was taken out, and placed in a -80°C refrigerator for 20 minutes to freeze, and a crystalline solid product was precipitated.
[0052] After the solid product is placed on filter paper to absorb excess water, it is placed in a vacuum oven to dry at room temperature to obtain a white crystalline blue fluorescent carbon quantum dot solid powder.
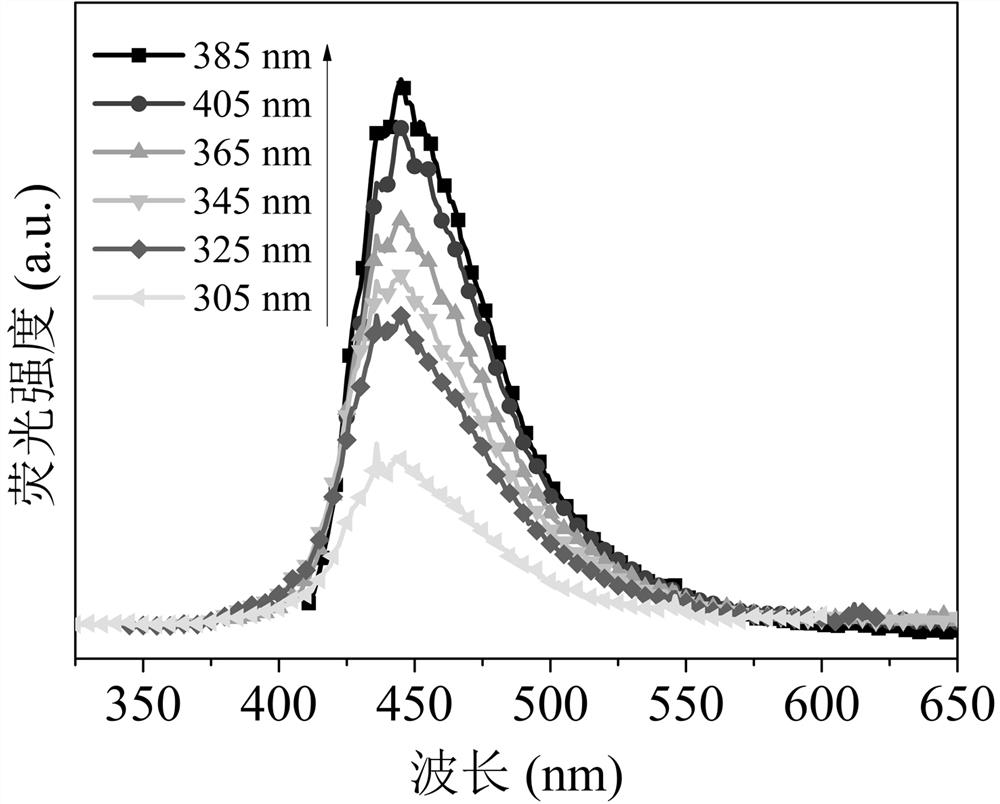
[0053] Figure 8 It is a fluorescence spectrum diagram of the prepared blue fluorescent carbon quantum dots at an excitation wavelength of 365nm, and the blue fluorescent carbon quantum dots have a strong blue fluorescent emission peak at 445nm.
[0054] F...
Embodiment 3
[0056] Add 1.6214 g of phloroglucinol dihydrate and 2.4024 g of urea into 10 mL of distilled water, and sonicate for 3 min to obtain a uniformly dispersed reaction solution.
[0057] The reaction solution was placed in a 100mL glass beaker and reacted in a 140W microwave oven for 15min. After the reaction was completed, it was cooled to room temperature, and the reaction solution was taken out, and left to stand at room temperature for 15 hours, and a crystalline solid product was precipitated.
[0058] After the solid product is placed on filter paper to absorb excess water, it is placed in a vacuum oven to dry at room temperature to obtain a white crystalline blue fluorescent carbon quantum dot solid powder.
[0059] Figure 10 It is a fluorescence spectrum diagram of the prepared blue fluorescent carbon quantum dots at an excitation wavelength of 365nm, and the blue fluorescent carbon quantum dots have a strong blue fluorescent emission peak at 460nm.
[0060] Figure 11...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com