Metformin hydrochloride transdermal delivery preparation and preparation method thereof
A metformin hydrochloride preparation technology, which is applied in the field of metformin hydrochloride transdermal preparations and its preparation, can solve the problems of stimulating lactic acid and large oral doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
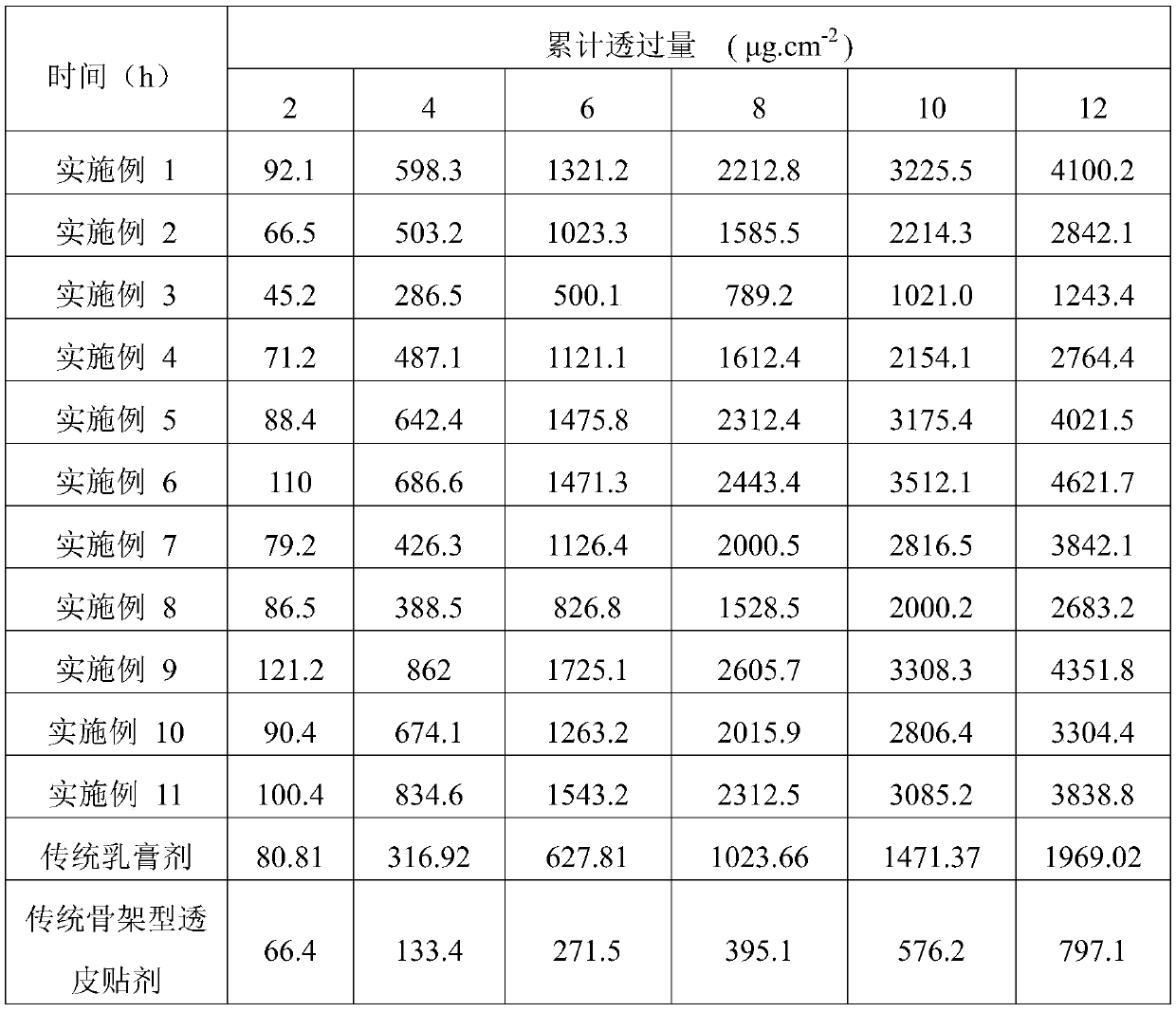
Examples
Embodiment 1
[0031] Embodiment 1 prepares the metformin hydrochloride liposome gel containing amphiphilic nano silicon dioxide
[0032] Metformin hydrochloride 50g, lecithin 90g, cholesterol 30g, amphiphilic nano silicon dioxide 10g, carbomer 15g, glycerin 5g, ethylparaben 2g, triethanolamine 0.25g, water for injection to 1000g, accurately weighed according to the above ratio Quantity, the preparation process is as follows:
[0033] 1) According to the ratio of lecithin: cholesterol: amphiphilic nano-silica: metformin hydrochloride = 9:3:1:5, take the above four substances and dissolve them in a small amount of ethanol; prepare an appropriate amount of phosphate buffer solution with a pH value of 6.8 , put it in a constant temperature water bath at 50°C, stir slowly, slowly inject the above mixture, stir for 2 hours, and obtain a liposome solution after the ethanol evaporates to dryness.
[0034] 2) Put the carbomer in an appropriate amount of phosphate buffer solution with an appropriate...
Embodiment 2
[0036] Embodiment 2 prepares the metformin hydrochloride liposome gel containing amphipathic hyaluronic acid
[0037] Metformin hydrochloride 50g, lecithin 90g, cholesterol 30g, hyaluronic acid 10g, carbomer 15g, glycerin 5g, ethylparaben 2g, triethanolamine 0.25g, add water for injection to 1000g, accurately weigh according to the above ratio, The preparation process is as follows:
[0038] 1) According to the ratio of lecithin: cholesterol: hyaluronic acid: metformin hydrochloride = 9:3:1:5, take the above four substances and dissolve them in a small amount of ethanol; prepare an appropriate amount of phosphate buffer solution with a pH value of 6.8, place In a constant temperature water bath at 50°C, stir slowly, slowly inject the above mixture, stir for 2 hours, and obtain a liposome solution after ethanol evaporates to dryness.
[0039] 2) Put the carbomer in an appropriate amount of phosphate buffer solution with an appropriate pH value of 6.8, let it fully swell overni...
Embodiment 3
[0041] Embodiment 3 prepares the metformin hydrochloride liposome gel containing amphipathic polyethylene glycol-polylactic acid-glycolic acid
[0042] Metformin hydrochloride 50g, lecithin 90g, cholesterol 30g, polyethylene glycol-polylactic acid-glycolic acid 10g, carbomer 15g, glycerin 5g, ethylparaben 2g, triethanolamine 0.25g, add water for injection to 1000g, press The above proportions are accurately weighed, and the preparation process is as follows:
[0043] 1) According to the ratio of lecithin: cholesterol: polyethylene glycol-polylactic acid-glycolic acid: metformin hydrochloride = 9:3:1:5, take the above four substances and dissolve them in a small amount of ethanol; configure a pH value of 6.8 Put an appropriate amount of phosphate buffer solution in a constant temperature water bath at 50°C, stir slowly, slowly inject the above mixture, stir for 2 hours, and obtain a liposome solution after the ethanol evaporates to dryness.
[0044] 2) Put the carbomer in an a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com