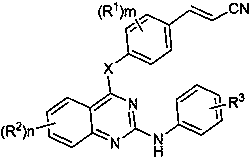
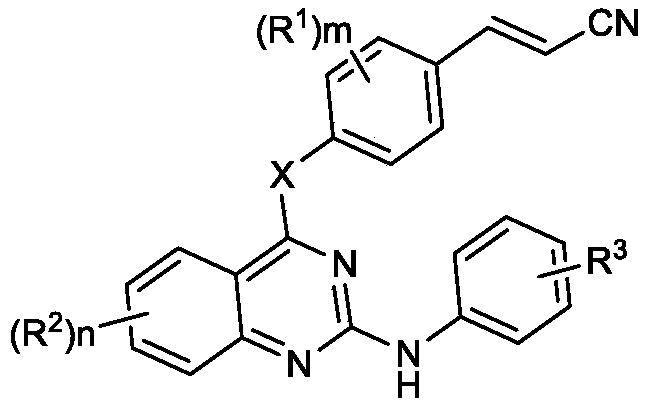
Cyano-vinyl substituted benzodiaryl pyrimidine compound and preparation method and application thereof
A technology of benzodiarylpyrimidines and compounds, which is applied in the field of benzodiarylpyrimidines and their preparation, can solve problems such as poor patient response rate, limited clinical use, and drug inactivation, and achieve low cytotoxicity, Significant anti-HIV-1 activity, the effect of improving biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
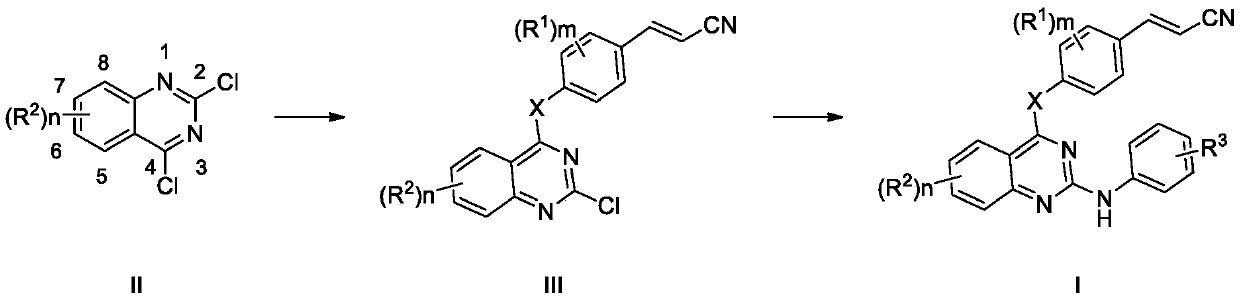
[0036] Embodiment 1: the synthesis of trans-3-(4-((2-chloroquinazoline-4-) amino)-3,5-dimethylphenyl)acrylonitrile
[0037]
[0038] Trans-3-(4-amino-3,5-dimethylphenyl)acrylonitrile (334.8mg, 10mmol), 2,4-dichloroquinazoline (258.7mg, 13mmol), palladium acetate (112mg, 0.5 mmol), 2-dicyclohexylphosphino-2'-(N,N-dimethylamine)-biphenyl (394mg, 1mmol), potassium phosphate (6.37g, 30mmol) in N,N-dimethylformamide (80 mL) was heated at 140° C. for 12 hours under nitrogen protection. TLC showed the reaction was complete. Diluted with ethyl acetate (10 mL), washed with saturated sodium carbonate solution (10 mL×2), water (10 mL×2), and saturated brine (10 mL×2) successively, and dried the organic phase over anhydrous sodium sulfate overnight. Filtration, concentration, and column chromatography gave the desired solid.
[0039] Yield 23%, white solid; 1 H NMR (400MHz, DMSO-d 6 )δ10.15(s,1H,NH),8.54(d,J=8.1Hz,1H,ArH),7.89(t,J=7.6Hz,1H,ArH),7.72(d,J=8.2Hz,1H ,ArH),7.68–7.58(m...
Embodiment 2
[0044] Example 2: trans-4-((6-chloro-4-((4-(2-cyanovinyl)-2,6-difluorophenyl)amino)quinazoline-2-)amino)benzene Nitrile Synthesis. The structural formula of this compound is:
[0045]
[0046] Compound II trans-3-(4-((2,6-dichloroquinazoline-4-)amino)-3,5-difluorophenyl)acrylonitrile (2mmol), p-aminobenzonitrile (4mmol, 472mg ) in n-butanol (5 mL) was refluxed for 6-8 hours. Insoluble matter was precipitated in the reaction solution, filtered, washed with dichloromethane (5mL×3), and dried to obtain compound I trans-4-((6-chloro-4-((4-(2-cyanovinyl)- 2,6-Difluorophenyl)amino)quinazoline-2-)amino)benzonitrile.
[0047] Yield 41%, white solid, melting point >325°C; 1 H NMR (400MHz, DMSO-d 6 )δ11.61(s,1H,NH),10.76(s,1H,NH),8.84(s,1H,ArH),8.07(s,2H,ArH),7.96(d,J=8.6Hz,1H, ArH), 7.82–7.69 (m, 2H, ArH and olefinic H), 7.53 (s, 4H), 6.81 (d, J=16.8Hz, 1H, olefinic H). 13 CNMR (101MHz, DMSO-d 6 )δ160.18, 153.38, 147.77, 142.99, 136.15, 135.04, 134.80, 133.15, 129.23, 128.31...
Embodiment 3
[0048] Example 3: trans-4-((6-chloro-4-((4-(2-cyanovinyl)-2-fluoro-6-methylphenyl)amino)quinazoline-2-)amino ) Synthesis of benzonitrile. The structural formula of this compound is:
[0049]
[0050] The operation method is the same as above. Yield 52%, yellow solid, melting point 278-282°C. 1 H NMR (400MHz, DMSO-d 6 )δ10.98(s,1H,NH),10.56(s,1H,NH),8.81–8.74(m,1H,NH),8.04–7.52(m,9H,Ar Handolefinic H),6.36(d,14.2Hz ,1H,olefinic H),2.31(s,3H,CH 3 ). 13 C NMR (101MHz, DMSO-d 6 )δ160.29, 157.06, 149.47 (d, J C-F =2.5Hz), 143.22, 139.19, 135.79, 134.84, 133.44 (d, J C-F =11.7Hz), 133.17, 129.01, 126.65 (d, J C-F =2.1Hz), 124.20, 120.54, 119.51, 118.96, 112.63 (d, J C-F =22.0Hz), 112.17, 99.10, 18.12 (d, J C-F =2.3Hz).HRMScalcd for C 25 h 16 ClFN 6 [M+H] + :455.1182,found:455.1168.HPLC analysis:t R = 19.25 min, 95.01%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com