Piceatannol oligomer, preparation method therefor and application of piceatannol oligomer
A piceatannol and oligomer technology, which is applied in the field of separation and purification and extraction of plant components, can solve the problems of difficult piceatannol oligomers and high polarity, and achieves high sensitivity, fast separation speed and high The effect of separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
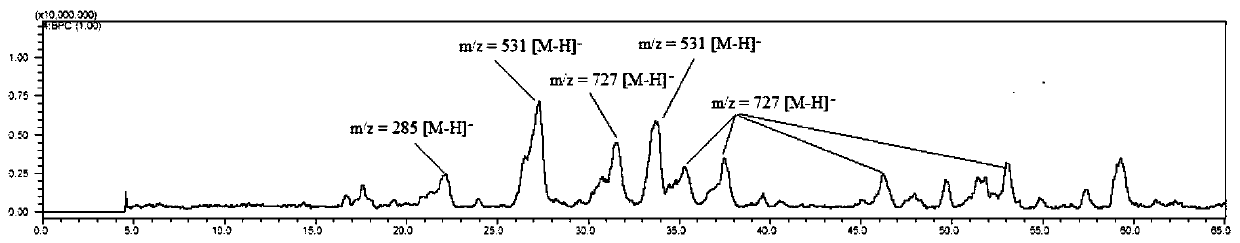
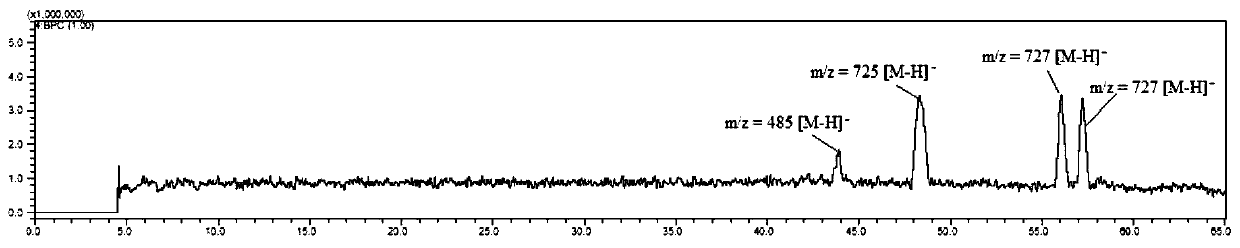
Examples
Embodiment 1
[0068] The preparation method of the piceatanol oligomer of the present embodiment, comprises the steps:
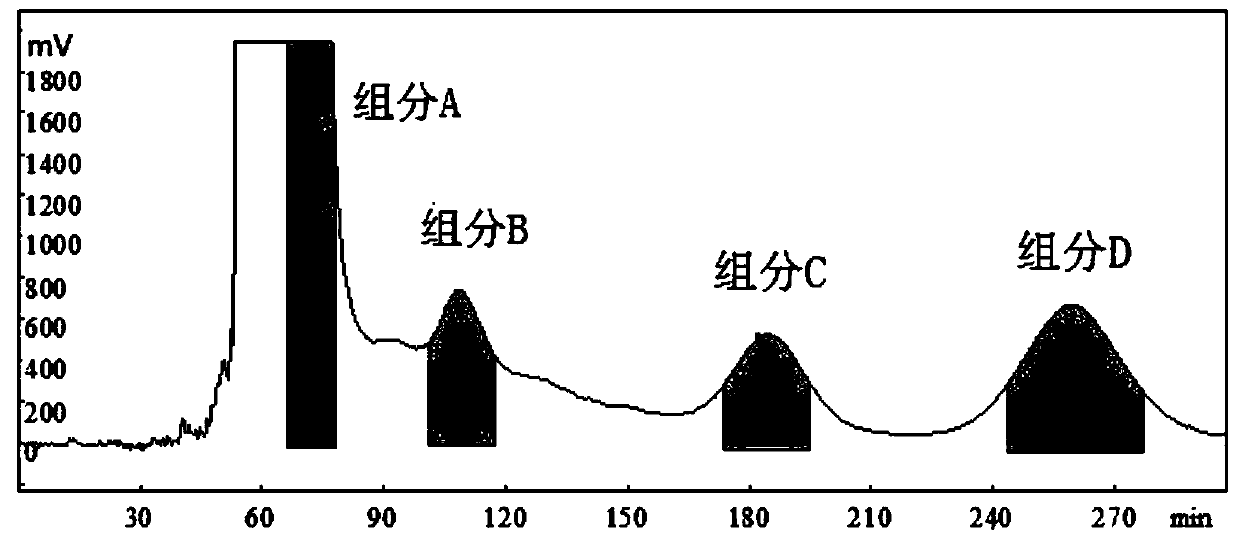
[0069] Step 1: High Speed Counter-Current Chromatography Separation
[0070] The separated two-phase solvent system is obtained by mixing n-hexane, ethyl acetate, methanol and water in a volume ratio of 10:20:10:28, and then standing to separate layers.
[0071] The upper phase of the two-phase solvent system is used as the stationary phase, and the lower phase is used as the mobile phase.
[0072] First inject the stationary phase into a high-speed countercurrent chromatography column (300mL) at a temperature of 25°C at a flow rate of 30mL / min. After the stationary phase is filled with the high-speed countercurrent mL / min into the high-speed countercurrent chromatography column.
[0073] After the dynamic equilibrium of the whole system is established, 72.5 mL of the stationary phase flows out at this time, and the retention rate of the stationary phase is S f The c...
Embodiment 2
[0121] The preparation method of the piceatanol oligomer of the present embodiment, comprises the steps:
[0122] Step 1: High Speed Counter-Current Chromatography Separation
[0123] The separated two-phase solvent system is obtained by mixing n-hexane, ethyl acetate, methanol and water in a volume ratio of 10:18:10:24, and then standing to separate.
[0124] The upper phase of the two-phase solvent system is used as the stationary phase, and the lower phase is used as the mobile phase.
[0125] First inject the stationary phase into a high-speed countercurrent chromatography column (300mL) at a temperature of 25°C at a flow rate of 30mL / min. After the stationary phase is filled with the high-speed countercurrent chromatography column, adjust the rotation speed to 800r / min, and then inject the mobile phase at a flow rate of 2.0 mL / min into the high-speed countercurrent chromatography column.
[0126] After the dynamic equilibrium of the whole system is established, 77.4 mL ...
Embodiment 3
[0136] The preparation method of the piceatanol oligomer of the present embodiment, comprises the steps:
[0137] Step 1: High Speed Counter-Current Chromatography Separation
[0138] The separated two-phase solvent system is obtained by mixing n-hexane, ethyl acetate, methanol and water in a volume ratio of 10:22:10:32, and then standing to separate.
[0139] The upper phase of the two-phase solvent system is used as the stationary phase, and the lower phase is used as the mobile phase.
[0140] First inject the stationary phase into a high-speed countercurrent chromatography column (300mL) at a temperature of 25°C at a flow rate of 30mL / min. After the stationary phase is filled with the high-speed countercurrent chromatography column, adjust the rotation speed to 900r / min, and then inject the mobile phase at a flow rate of 2.0 mL / min into the high-speed countercurrent chromatography column.
[0141] After the dynamic equilibrium of the whole system is established, 76.5 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com