Pharmaceutical composition containing indirubin derivative as active ingredient
一种组合物、靛玉红的技术,应用在含有效成分的医用配制品、药物组合、有机活性成分等方向,能够解决昂贵、注射施用麻烦等问题,达到提高骨厚度、提高骨长度、优异预防或治疗作用的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
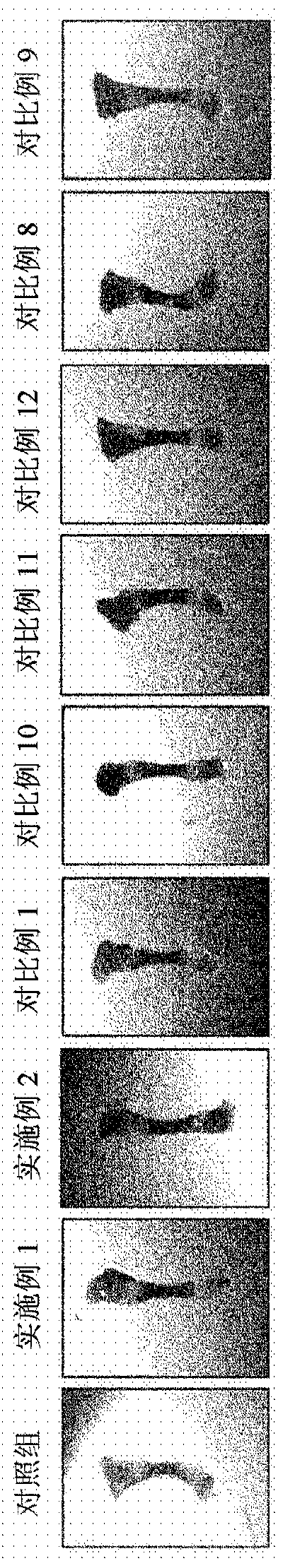
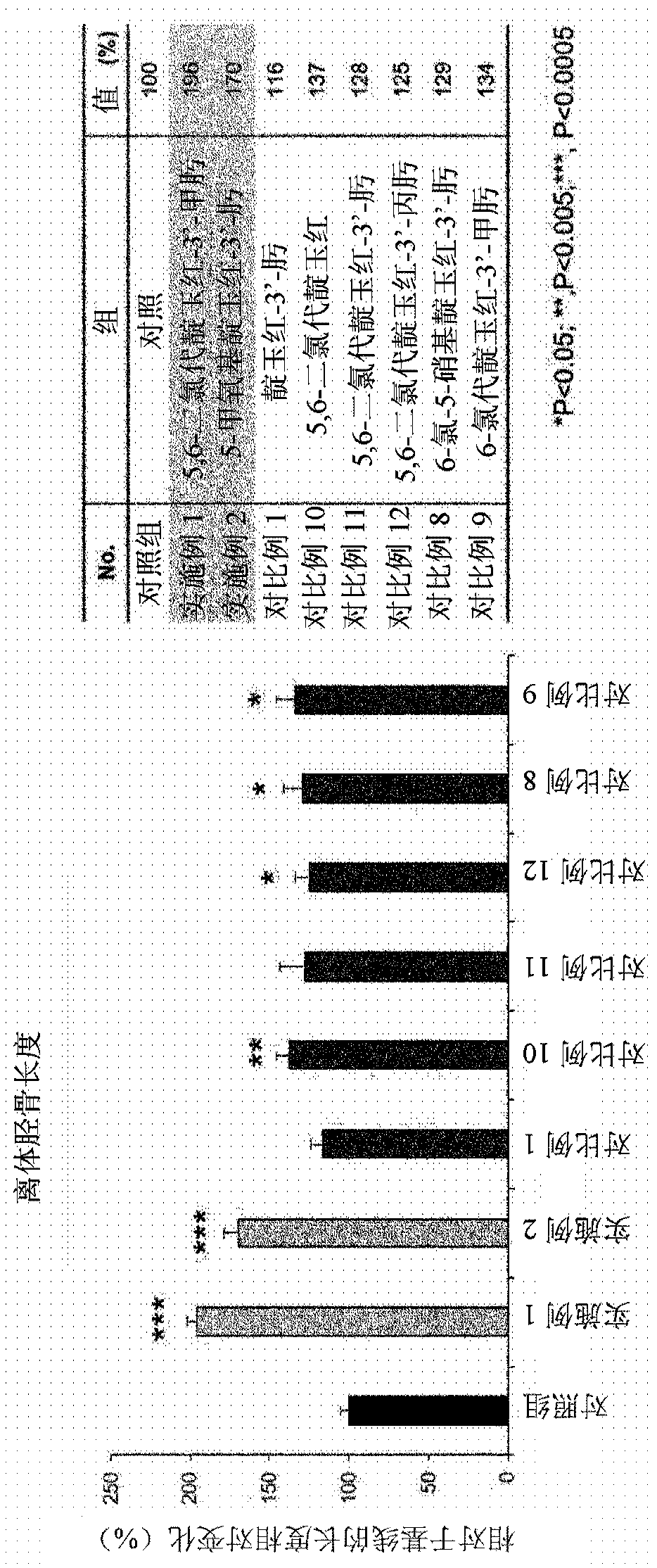
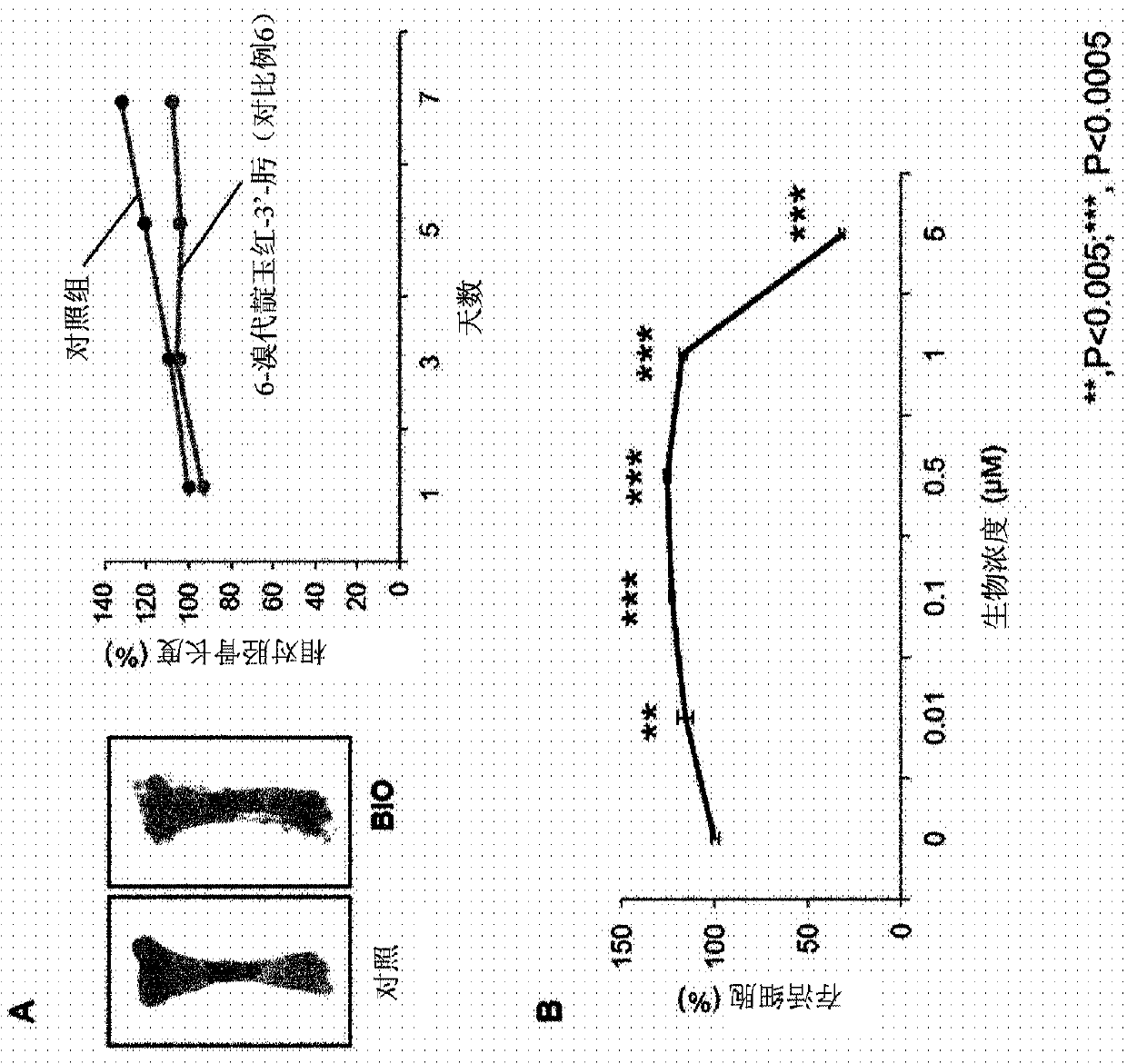
Image
Examples
Embodiment 1
[0081] Example 1. Synthesis of 5,6-dichloroindirubin-3'-methoxime (A3051)
[0082] ①Synthesis of intermediate 5',6'-dichloro-[2,3'-dihydroindolylidene]-2'3-dione
[0083]
[0084] 5,6-Dichloroisobutane (500mg, 2.32mmol) was added to a 250mL round bottom flask and dissolved in MeOH (92.80ml) and indoxyl acetate (405.48mg, 2.315mmol) and Sodium carbonate (Na 2 CO 3 ) (637.83mg, 6.02mmol) was stirred at 65°C for 12 hours. Termination of the reaction was checked using TLC (Rf = 0.4; ethyl acetate / hexane = 1 / 2 (v / v)), and the product was cooled until crystalline material formed in ice. When crystals formed, they were filtered off, the solvent was removed, the filtrate was discarded and the product was washed several times with solvent (ethanol / water=1 / 1 (v / v)). The product was filtered to dry in vacuum pump and used in the next step without further purification.
[0085] ② Synthesis of A3051
[0086]
[0087] 5'6'-dichloro-[2,3'-dihydroindolylidene]-2'3-dione (5',6'-dichl...
Embodiment 2
[0089] Example 2. Synthesis of 5-methoxyl indirubin-3'-oxime (A3334)
[0090] ① Synthesis of intermediate 5'-methoxy-[2,3'-dihydroindolinylidene]-2',3-dione
[0091]
[0092] 5-Methoxyisatin (1000 mg, 5.65 mmol) was added to a 250 ml round bottom flask and dissolved in MeOH (225 ml). Add indole acetate (989mg, 5.65mmol) and sodium carbonate (Na 2 CO 3 ) (1496mg, 14.11mmol) was stirred at 65°C for 12 hours. Termination of the reaction was checked using TLC (Rf = 0.4; ethyl acetate / hexane = 1 / 2 (v / v)), and the product was cooled until crystalline material formed in ice. After crystals formed, they were filtered off, the solvent was removed, the filtrate was discarded, and the product was washed several times with solvent (ethanol / water=1 / 1 (v / v)). The resulting water was filtered to dry in a vacuum pump and used in the next step without further purification.
[0093] ②Synthesis of A3334
[0094]
[0095] 5'-methoxy-[2,3'-dihydroindolylidene]-2',3-dione (670mg, 2.29mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com