A CRISPR-Cas system for diagnosing spinal muscular atrophy and its application
A technology for spinal muscular atrophy and diagnostic kits, which is applied in the determination/testing of microorganisms, DNA/RNA fragments, recombinant DNA technology, etc., and can solve the problem of expensive detection costs, unsuitable for large-scale promotion and use, and long detection cycles, etc. problem, to achieve the effect of low cost, high accuracy rate, high specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] An embodiment of the spinal muscular atrophy diagnostic kit of the present invention, the kit includes the following components: FnCas12a, crRNA, dsDNA and reporter RNA strand; the crRNA sequence is shown in SEQ ID No.1; The dsDNA amplification primers include an upstream primer shown in SEQ ID No.2 and a downstream primer shown in SEQ ID No.3; the reporter RNA chain sequence is shown in SEQ ID No.4, and the reporter RNA chain The 5' end is modified with a fluorescent group FAM, and the 3' end is modified with a quenching group BHQ1.
[0028] SEQ ID No.1: 5'-GAAATTAATACGACTCACTATAGGGTAATTTCTACTAAGTGTAGATAGACAAAATCAAAAAGAAGG-3';
[0029] SEQ ID No.2: 5'-TATAAAGCTATCTATATA-3';
[0030] SEQ ID No. 3: 5'-AGGTGCTCACATTCCTTAAATTAA-3'.
[0031] SEQ ID No. 4: 5'FAM-TTATT-BHQ1 3'.
[0032] The crRNA preparation method comprises the following steps: (1) designing and synthesizing the crRNA whose sequence is shown in SEQ ID No.1; (2) transcribing the crRNA obtained in step (1) ...
Embodiment 2
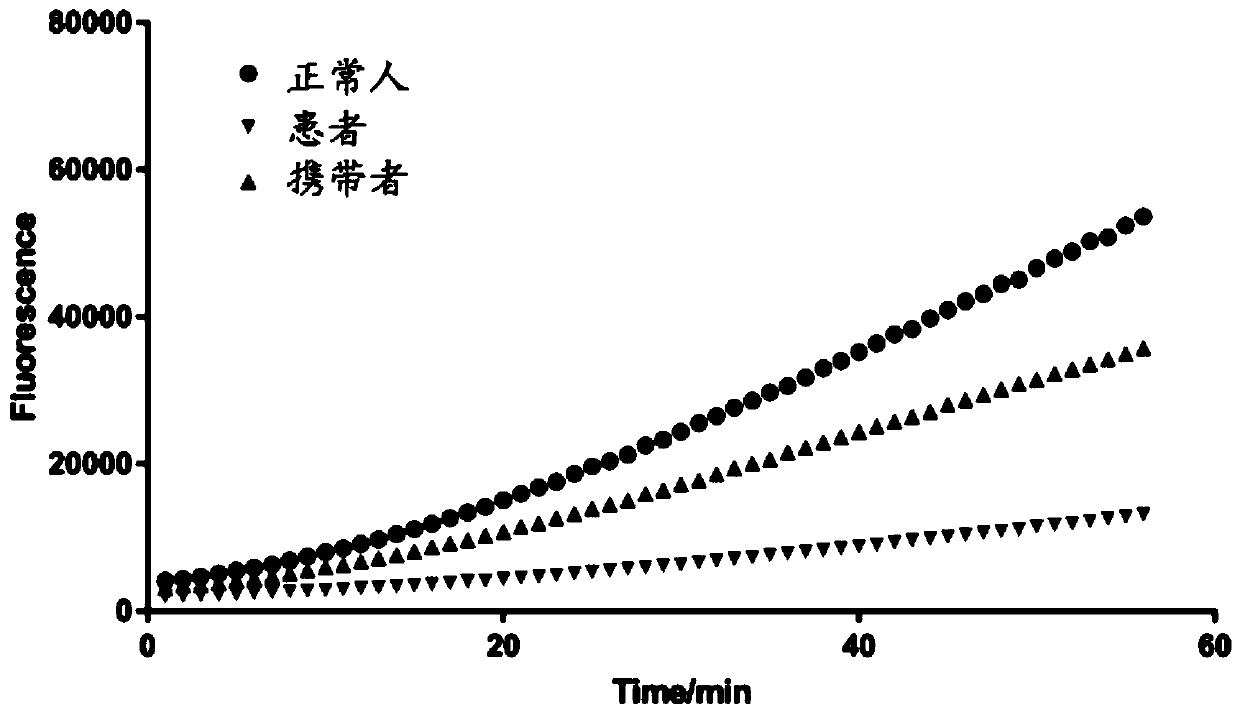
[0038] In this example, the kit described in Example 1 was used to detect 20 suspected spinal muscular atrophy patient samples, and at the same time compared with the final clinical diagnosis results.
[0039] 1. Detection method
[0040] 1. Use the DNA extraction kit to extract the DNA of the patient's saliva sample, and the operation steps are strictly in accordance with the instructions;
[0041] 2. Prepare dsDNA corresponding to each sample according to the dsDNA preparation method described in Example 1;
[0042]3. Configure the following reaction system (100uL): FnCas12a 45nM, crRNA 67.5nM, dsDNA 100ng, reporter RNA strand 125nM.
[0043] 4. Incubate the FnCas protein and crRNA at 37°C for 10 minutes before using the machine, and detect the fluorescence every 1 minute on a microplate reader.
[0044] 2. Test results
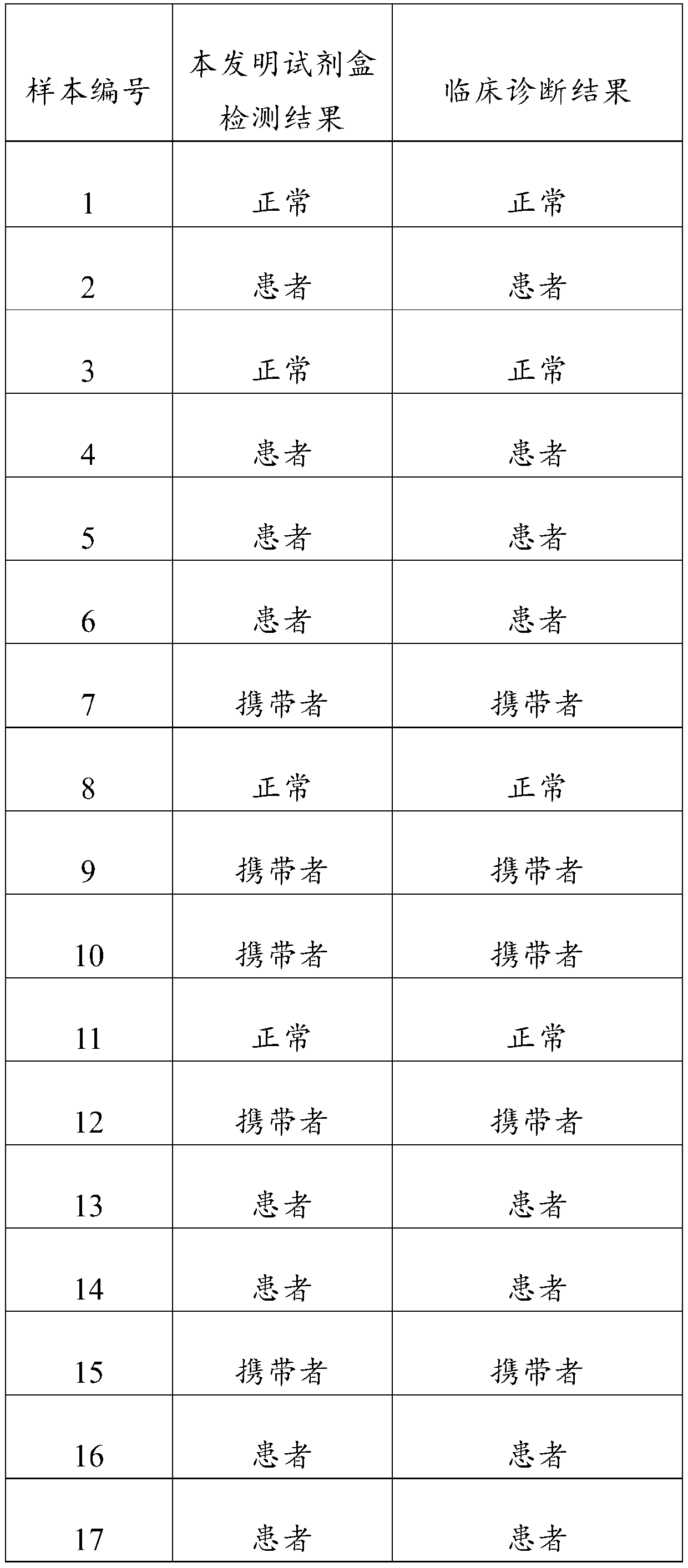
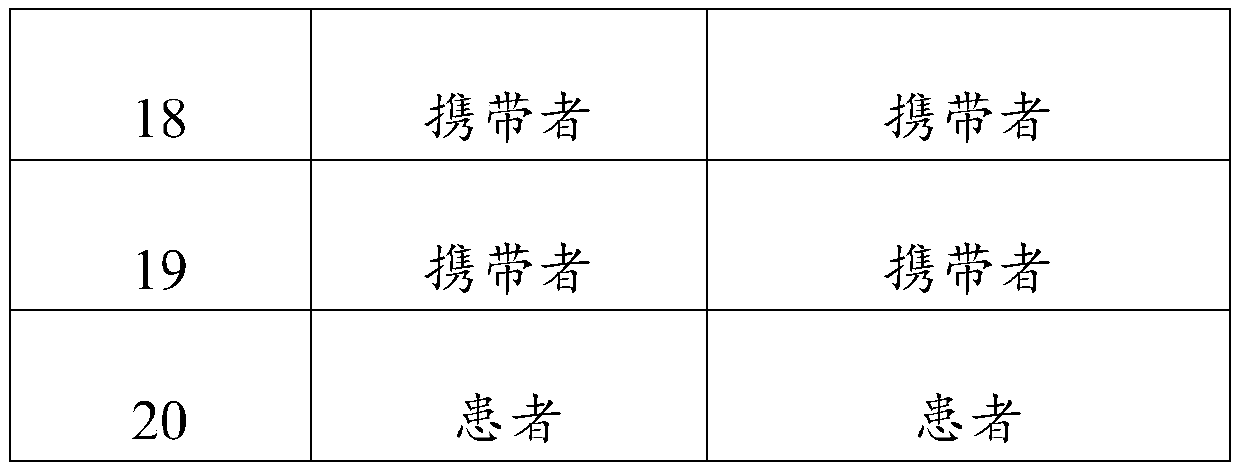
[0045] The test results are shown in Table 1:
[0046] Table 1 Test results of 20 samples
[0047]
[0048]
[0049] From the above results, it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com