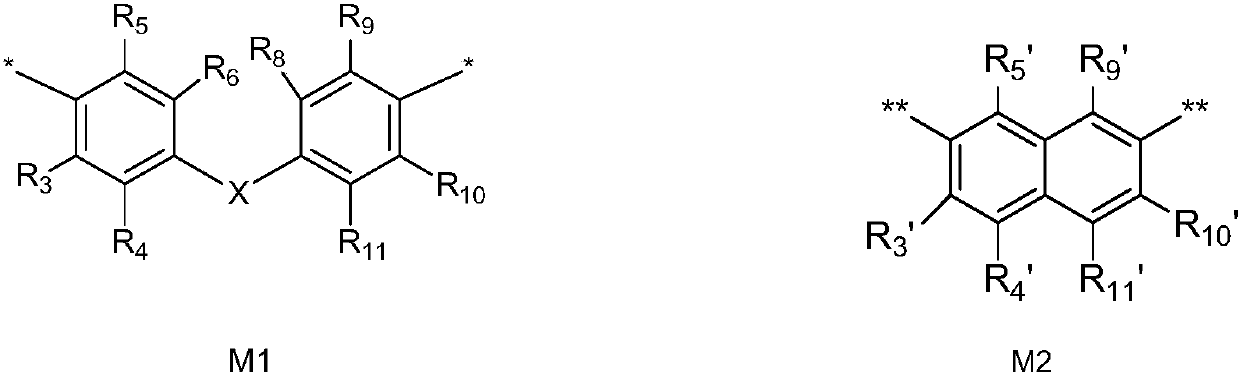Mono/bis-methyl cinnamate photoinitiators as well as preparation method and application thereof
A technology of methyl cinnamate and group, which is applied in the preparation and application field of the mono- or di-methyl cinnamate compounds, and can solve the problems of strong mobility, low price, bad smell, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
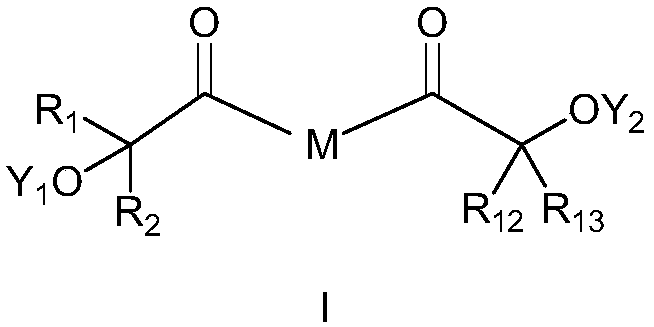
[0124] Example 1: Bis[4-(2-hydroxy-2-methylpropionyl)-phenyl]methane dicinnamate (compound 1)
[0125]
[0126] Put 34g (0.1mol) of bis[4-(2-hydroxy-2-methylpropionyl)-phenyl]methane into a 250ml three-necked flask, add 100ml of dichloromethane and 2.4g (0.1mol) of sodium hydride. After stirring evenly, 33.2 g (0.2 mol) of 3-phenyl-2-acryloyl chloride was added, and the reaction was stirred at room temperature for 3 h. Then, filter the reaction solution, wash the filtrate successively with dilute hydrochloric acid (5%), saturated aqueous sodium bicarbonate solution and distilled water, then dry overnight with anhydrous sodium sulfate and filter, remove the organic phase by distillation under reduced pressure, and recrystallize methanol to obtain Pale yellow crystals, 48 g in total, 80% yield. The product has been 1 H-NMR spectrum confirmed the title compound.
[0127] 1 H-NMR (spectrum) (measured in Acetone-d6) (δppm): 1.60 (s, 12H), 3.96 (s, 2H), 6.31 (d, 2H), 6.78 (...
Embodiment 2
[0128] Example 2: 2-hydroxy-1-(4-(4-(1-hydroxycyclohexanecarbonyl)phenoxy)phenyl)-2-methyl-1-propanone monocinnamate (compound 2)
[0129]
[0130] Put 38.2g (0.1mol) of 2-hydroxy-1-(4-(4-(1-hydroxycyclohexanecarbonyl)phenoxy)phenyl)-2-methyl-1-propanone in a 250ml three-necked flask , 100 ml of dichloromethane and 10.1 g (0.1 mol) of triethylamine were added. After stirring evenly, 16.6 g (0.1 mol) of 3-phenyl-2-acryloyl chloride was added, and the reaction was stirred at room temperature for 3 h. Then, filter the reaction solution, wash the filtrate successively with dilute hydrochloric acid (5%), saturated aqueous sodium bicarbonate solution and distilled water, then dry overnight with anhydrous sodium sulfate and filter, remove the organic phase by distillation under reduced pressure, and recrystallize methanol to obtain Pale yellow crystals, 38 g in total, yield: 74.2%. The product has been 1 H-NMR spectrum confirmed the title compound.
[0131] 1 H-NMR (spectrum)...
Embodiment 3
[0132] Example 3: Bis[4-(2-hydroxy-2-methylpropionyl)phenyl]sulfide monocinnamate (compound 3)
[0133]
[0134] Put 35.8g (0.1mol) of bis[4-(2-hydroxy-2-methylpropionyl)phenyl]sulfide in a 250ml three-necked flask, add 100ml of dichloromethane and 2.4g (0.1mol) of sodium hydride. After stirring evenly, 16.6 g (0.1 mol) of cinnamoyl chloride was added, and the reaction was stirred at room temperature for 3 h. Then, filter the reaction solution, wash the filtrate successively with dilute hydrochloric acid (5%), saturated aqueous sodium bicarbonate solution and distilled water, then dry overnight with anhydrous sodium sulfate and filter, remove the organic phase by distillation under reduced pressure, and recrystallize methanol to obtain Pale yellow crystals, 36 g in total, yield: 73.7%. The product has been 1 H-NMR spectrum confirmed the title compound.
[0135] 1 H-NMR (spectrum) (measured in Acetone-d6): 1.78 (s, 6H), 2.34 (s, 6H), 6.54 (d, 1H), 7.35 (m, 13H), 8.11 (d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com