Preparation process of moxifloxacin
A preparation process, moxifloxacin technology, applied in the field of moxifloxacin preparation process, can solve the problems of boron impurity difficult to remove, moxifloxacin purity and yield reduction, etc., to achieve the effect of high purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation technology of moxifloxacin comprises the following steps:
[0040] S1, synthesize MX-2;
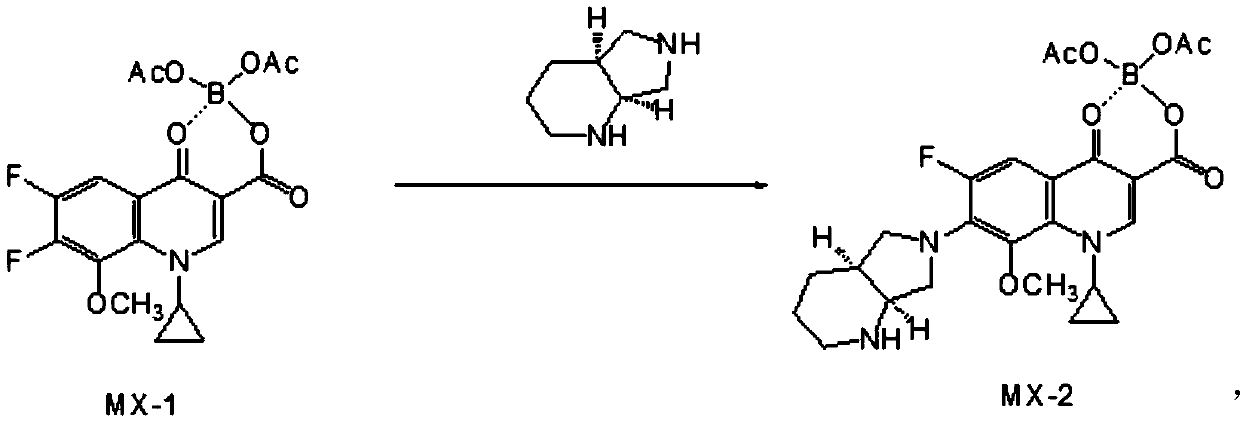
[0041] First, synthesize MX-2 according to the following formula:
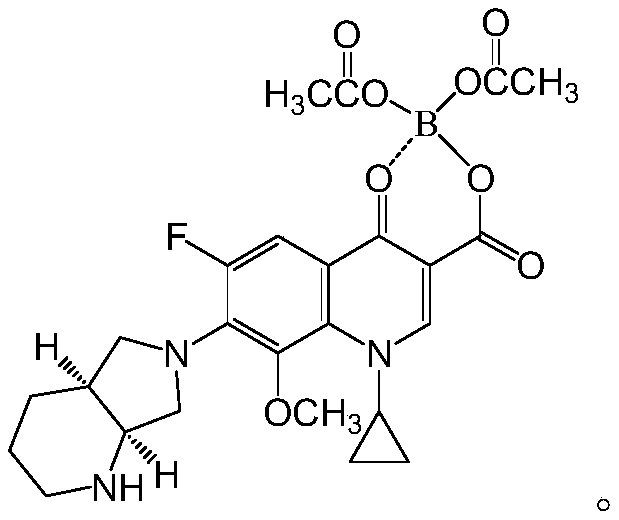
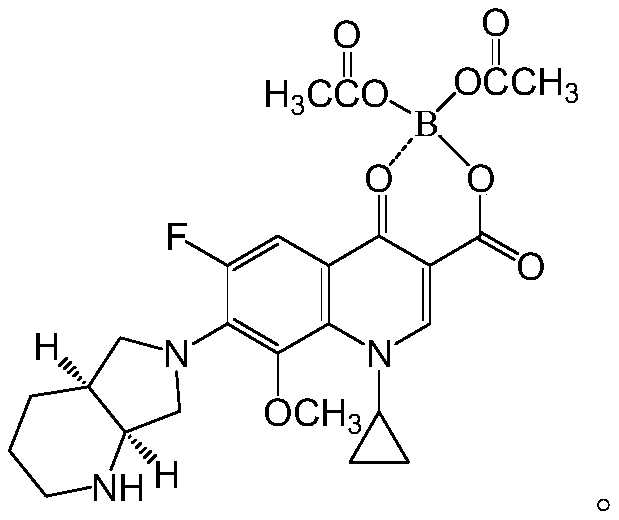
[0042]
[0043] Among them, the synthesis of MX-1 is a compound known to those skilled in the art, and its synthesis is also known to those skilled in the art, so the present invention will not describe it in detail.
[0044] Preferably, the synthesis includes: mixing and reacting MX-1, solvent, basic substance and (S,S)-2,8-diazabicyclo[4,3,0]nonane;
[0045] Preferably, the conditions of the synthesis reaction are: the temperature is 0-80°C, and the time is 30 minutes-20 hours;
[0046] More preferably, the temperature is 40-50° C. and the time is 1-1.5 hours.
[0047] Further, the molar ratio of MX-1 and alkaline substance is 1:0.2-3; more preferably 1:0.5-1;
[0048] Preferably, the alkaline substance is an amine substance, more preferably, triethylamine;
[0049] Preferably, the solvent...
Embodiment 1
[0084] The present embodiment provides a kind of preparation technology of moxifloxacin:
[0085] Prepare with reference to the following formula:
[0086]
[0087] Add 30.00g (0.073mol) MX-1 to a 500ml four-neck flask, add 74.5ml acetonitrile, stir until dissolved; slowly add 9.84g (0.075mol) (S,S)-2,8-diazabicyclo[4 ,3,0] nonane, triethylamine 10g (0.099mol), temperature control 45 ℃, stirring for 1.5 hours. Cool down to 0°C after the reaction and add cooling methanol-hydrochloric acid solution (74g (2.31mol) of methanol, 20.2g (0.204mol) of concentrated hydrochloric acid with a mass concentration of 37%)) and stir at 8°C for 10 minutes under temperature control, then add 20g of glycerol ( 0.217mol) and stirred for 10 minutes, added moxifloxacin with a purity of 99.5% as crystal form 2 as seed crystal, and stirred and grown the crystal for 1 hour, added acetone 108g (1.86mol) and continued stirring and grown the crystal for 3 hours; filtered and washed the filter cake wi...
Embodiment 2- Embodiment 9
[0089] Example 2-Example 9 Prepare moxifloxacin with reference to the preparation process provided in Example 1, the difference is that the specific operating conditions are different.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com