Minicircle DNA vaccine design and application
A technology of pathogenic microorganisms and antigens, applied in the design and application of microcircle DNA vaccines, can solve the problems of DNA vaccines such as low immunogenicity, low transfection efficiency, and limited antigenic protein, so as to enhance immunogenicity and avoid safety problems , the effect of efficient expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, minicircle DNA parent plasmid construction
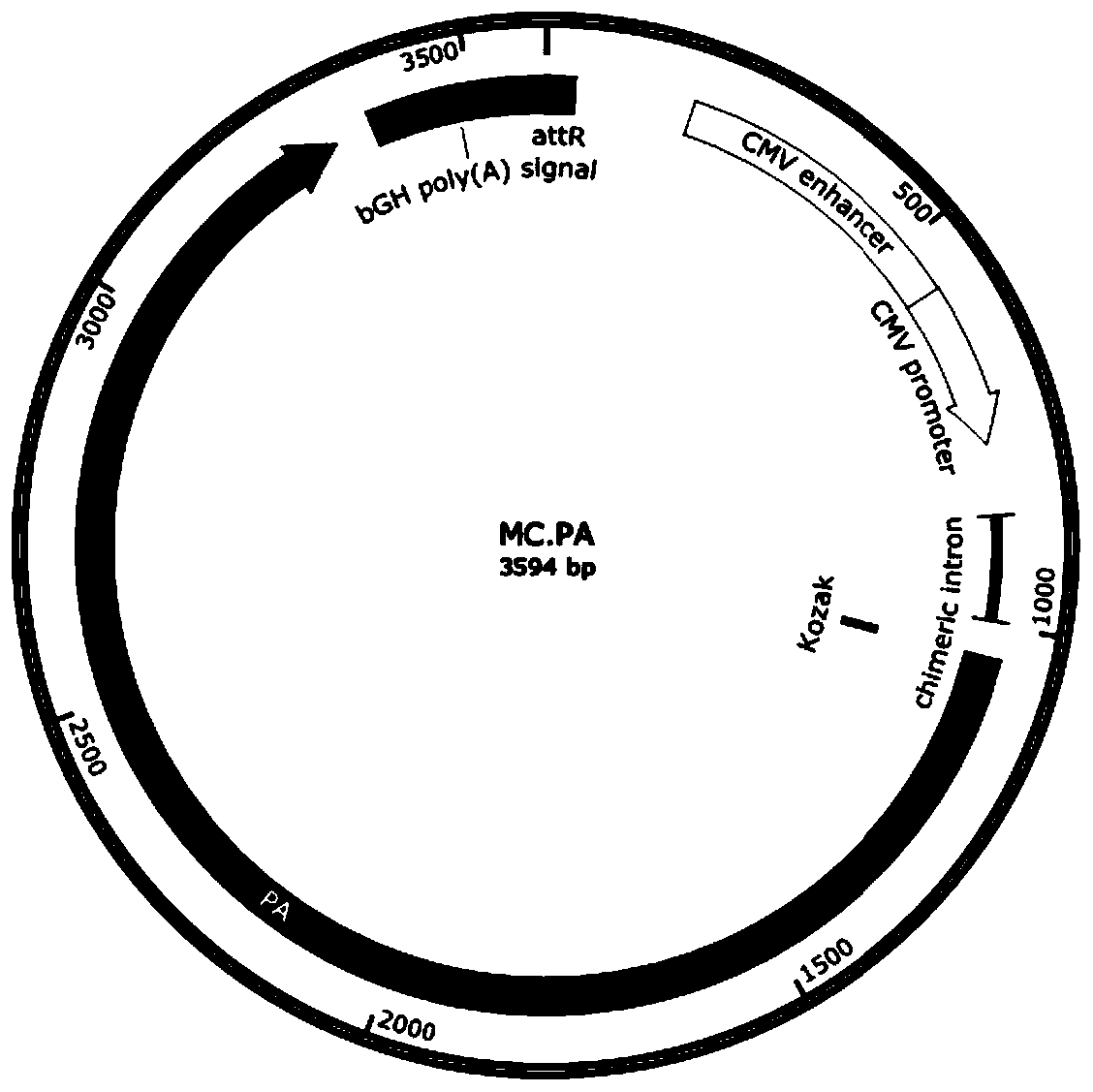
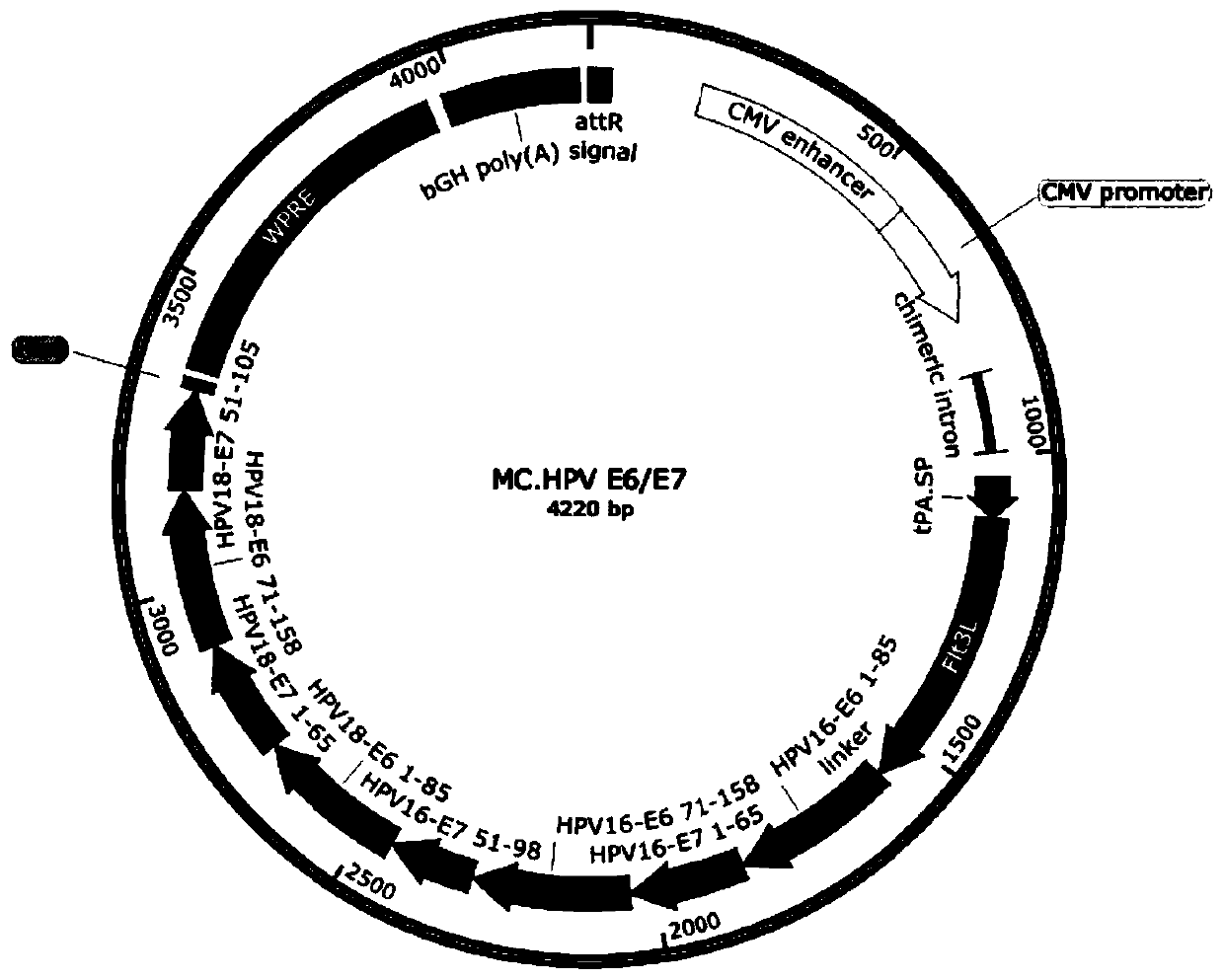
[0039] 1) Synthesizing a full-length target gene DNA fragment, the target gene is selected from coding gene sequences such as Bacillus anthracis PA, HPV E6 / E7 chimeric protein, HPV L1 protein, HPV L2 protein or HPV L1 / L2 chimeric protein (such as SEQ ID NO : 7-11).
[0040] 2) Subcloning the target gene fragment into the attB and attP sites of the minicircle DNA empty vector to construct the minicircle DNA master plasmid. The minicircle DNA parent plasmid contains attB and attP recombination sites, 32 tandem repeated I-SceI restriction sites (I-SceI*32), kana resistance gene, SV40 DNA nuclear targeting sequence (DNA nuclear targeting sequence, DTS), CMV promoter / enhancer, chimeric intron, target gene, bovine growth hormone (bGH) poly A signal.
Embodiment 2
[0041] Embodiment 2, microcircle DNA preparation
[0042] 1) The minicircular DNA master plasmid was transformed into genetically engineered E coli bacteria ZYCY10P3S2T (Nature Biotechnology 2010, 28:1287-9).
[0043] 2) Inoculate the ZYCY10P3S2T containing the minicircle master plasmid into the TB medium containing kana, culture it on a shaker at 37°C for 12-16h, and the bacteria will produce a large amount of minicircle DNA master plasmid.
[0044] 3) Add the induction medium containing arabinose, and under the induction of arabinose (32° C. shaker culture for 8 h), ZYCY10P3S2T expresses the ΦC31 recombinase and the endonuclease that recognizes the I-SceI site. Under the action of ΦC31 recombinase, the minicircle DNA parent plasmid undergoes DNA recombination at the attB / attP recombination site to form two small circular DNA molecules: i) minicircle DNA (only contains the target gene expression cassette and 36-bp attR site); ii) a small circle composed of plasmid backbone D...
Embodiment 3
[0046] Embodiment 3, Western Blot detection protein (Bacillus anthracis PA antigen) expression
[0047] 1. Experimental steps
[0048] 1) Prepare protein samples: use liposome transfection reagent (such as Lipofectamine 2000) to transfect microcircle DNA or equimolar microcircle DNA master plasmid into 293T cells, replace serum-free cell culture medium, and culture in serum-free medium Three days later, the culture supernatants of 293T cells were collected respectively.
[0049] 2) Electrophoresis: After adding an appropriate amount of loading buffer to the protein sample, heat it in boiling water for 3-5 minutes to denature the protein. After cooling, add the sample to the sample well of the SDS-PAGE gel, and electrophoresis at 80-100V for 1 hour.
[0050] 3) Membrane transfer: transfer the protein from the SDS-PAGE gel to the PVDF membrane using a wet transfer device (Bio-Rad, USA) at 300 mA for 1 hour.
[0051] 4) Blocking: After washing the PVDF membrane, add Western Blo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com