Combined diagnosis paper-based micro-fluidic chip and detection method
A microfluidic chip and paper-based technology, which is applied in the field of biomedical detection, can solve the problems of complex auxiliary sample injection structure and large instrument volume, and achieve the effects of saving detection time, simplifying detection steps, and great practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
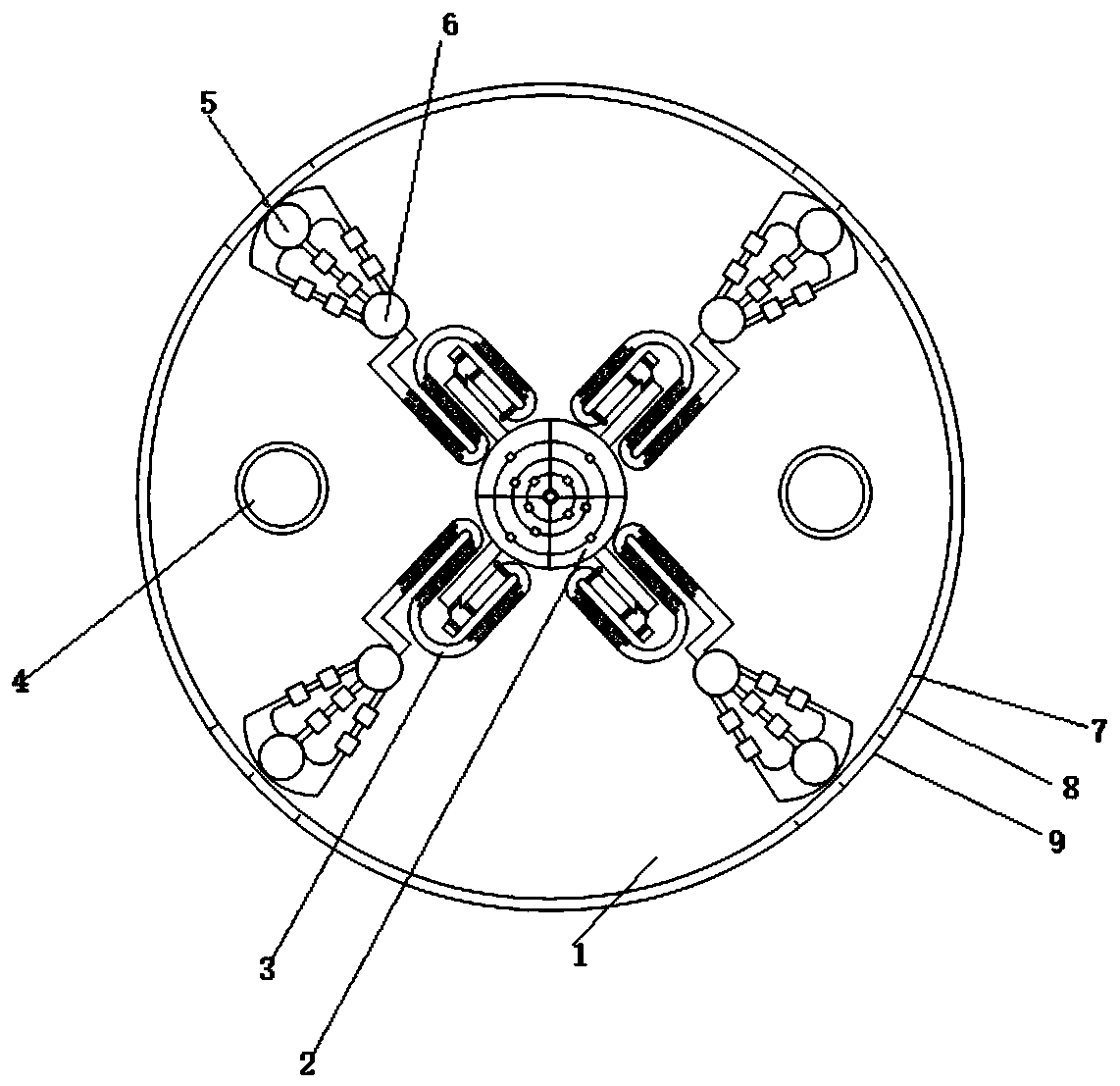
[0060] The invention provides a combined diagnostic paper-based microfluidic chip. It is mainly composed of various functional areas of the paper base (7), and the sample feeding part (2) and the sample dividing part (6). The chip (1) is disc-shaped, and the paper base (7) and the The chip substrate (9) of the paper base (7) and the sealing film (8) on the paper base (7) have a total of three layers; a round hole is set at the center of the chip (1), and the sampling component is placed in the round hole (2), more than 2 sample processing areas (3) are uniformly and symmetrically arranged along the diameter extension line around the sample inlet part (2); each sample processing area (3) is connected to 1 at the outlet along the diameter extension line Each sampling part (6); each sampling part is connected to a detection area (5); the chip (1) is also provided with more than two screw holes (4) along the direction of the extension line of the diameter for fixing;
[0061] The...
Embodiment 2
[0069] The invention provides a detection method.
[0070] Full quantitative detection of D-dimer (D-Dimer), troponin I (cTnI), myoglobin (MYO), creatine kinase isoenzyme (CKMB), C Reactive protein (CRP), procalcitonin (PCT), the contents of a total of 6 protein markers to be detected.
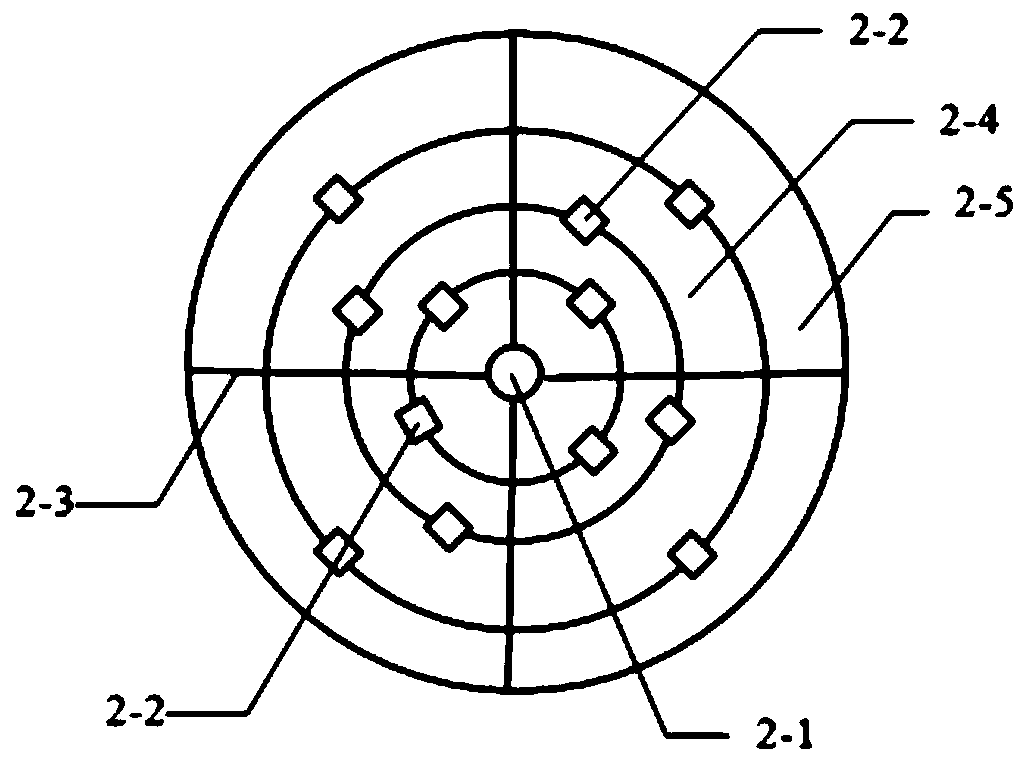
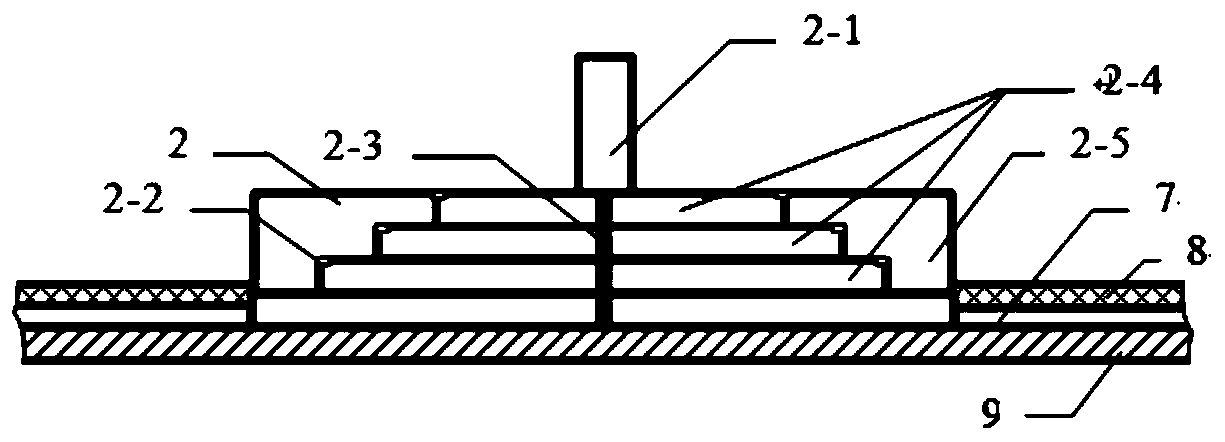
[0071] The first step, preparation: choose a chip 1 with 4 sedimentation equalization pools 2-4 and detection areas 5, and each detection area 5 has 2 detection channels 11. Label quantum dots on D-dimer (D-Dimer), troponin I (cTnI), myoglobin (MYO), creatine kinase isoenzyme (CKMB), C-reactive protein (CRP), calcitonin Primogen (PCT) mouse monoclonal antibody, two by two groups are sprayed in the nanofiber hole 3-9 of quantum dot reaction channel 3-3 before a detection area 5; The anti-antibody is sprayed in the detection reaction chamber 5-1 and the quality inspection reaction chamber 5-2 of the detection channel 11 of the same detection area 5, and dried at 37 degrees. Install the sampli...
Embodiment 3
[0077] Test results
[0078] According to the detection method and steps of Example 2, the following experimental results were obtained.
[0079] The coefficients of variation of the six target markers ranged from 5.20% to 8.30%, the lowest detection limit of which was 0.26ng / mL, and the correlation coefficient r≥0.9992 within the linear range of 2.50ng / mL to 108.00ng / mL.
[0080] The test results of creatine kinase isoenzymes were tested by the same batch of whole blood samples as those in the hospital, and the experimental data were compared with the original test results of the hospital one by one. The linear correlation fitting equation is y=1.0050x+0.1098, and the correlation coefficient r = 0.9970, ie R 2 = 0.9940. It shows that there is no significant difference between the creatine kinase isoenzyme test results and the hospital test results. See Figure 11 .
[0081] The linear correlation fitting equation between C-reactive protein test results and hospital measu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
| coefficient of variation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com