Sialic acid lipid derivative and preparation method and application thereof
A technology of lipid derivatives and sialic acid, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems that carboxyl groups cannot be exposed and are unfavorable, so as to improve placement stability, activity and selection sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] The synthesis of embodiment 1 methyl sialate (MT)
[0104] Add SA (20g, 0.064mol), methanol 250mL, and 3.68N methanolic HCl solution 1.7mL (0.0064mol) into a 500mL three-necked flask, and stir the reaction in an oil bath at 50°C for 2.5h. After suction filtration, the filtrate was evaporated to dryness to obtain 19.3 g of white solid, which was recrystallized by adding 80 mL of methanol, cooled and crystallized, and 17.90 g of white solid was obtained after suction filtration, with a yield of 86.5%.
[0105] The structural formula is as follows:
[0106]
[0107] Melting point: 182-183°C.
[0108] Mass Spectrum: m / z 346.1, MT[M+Na] + peak; no SA peak.
[0109] The SAs in the examples all take Neu5Ac as an example. Under the same reaction conditions, the corresponding products can be obtained by using Neu5Gc and KDN in the same molar ratio instead of Neu5Ac.
Embodiment 2 18
[0110] The synthesis of embodiment 2 octadecanoyl chloride
[0111] Add octadecanoic acid (8.53 g, 0.03 mol) and thionyl chloride (28.56 g, 0.24 mol) into a 100 mL eggplant-shaped bottle, and react under reflux in an oil bath at 80° C. for 1 h. The remaining thionyl chloride was distilled off under reduced pressure at 65°C to obtain 8.9 g of orange liquid with a yield of 98.2%. The acid chlorides used in the remaining examples were all synthesized under the same reaction conditions and the same molar ratio.
[0112] The structural formula is as follows:
[0113]
Embodiment 3
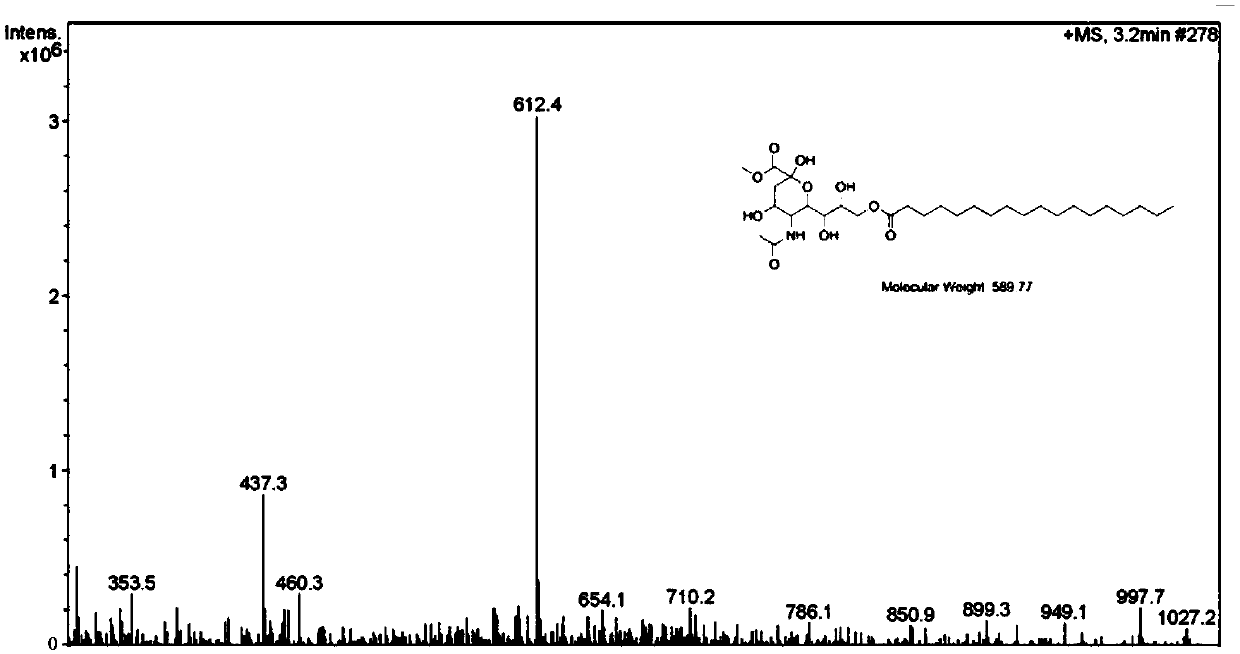
[0114] Synthesis of embodiment 3 MT-18 (compound 3)
[0115] Add MT (5g, 15mmol) and 4-dimethylaminopyridine (0.19g, 1.5mmol) to a 250mL three-necked flask, dissolve it with 50mL of pyridine, and add octadecanoyl chloride (7.1g, 23mmol) dropwise under an ice bath. THF solution 40mL, control the temperature at about 5°C, move to room temperature after 20min to continue the reaction, add 50mL of water after 10h of reaction, extract with 40mL of dichloromethane*3, wash with 30mL of saturated saline, dry with anhydrous MgSO4, pump Filtration, spin-drying, sample mixing, column packing, separation by silica gel column chromatography to obtain 2.82 g of white solid, yield: 55.6%.
[0116] The structural formula is as follows:
[0117]
[0118] Melting point: 140-142°C.
[0119] Mass Spectrum: m / z:612.4, MT-18[M+Na] + peak. Spectrum see attached figure 1 .
[0120] Proton nuclear magnetic resonance spectrum: 1H-NMR (600MHz, DMSO-d6): δ8.12 (d, J = 8.4Hz, 1H), 6.38 (s, 1H), 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com