Novel matrix sustained-release tablet and preparation method thereof
A skeleton slow-release material and slow-release tablet technology, applied in the field of medicine, can solve the problems of accelerated drug release, slow release rate, single release mechanism, etc., and achieve the effect of increasing the release rate and uniform drug release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1 Venlafaxine Hydrochloride Ordinary Matrix Sustained Release Tablet
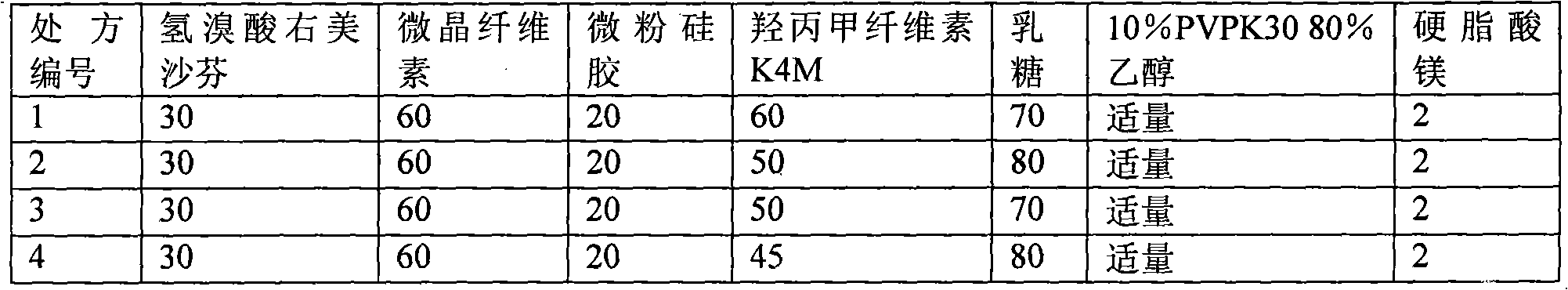
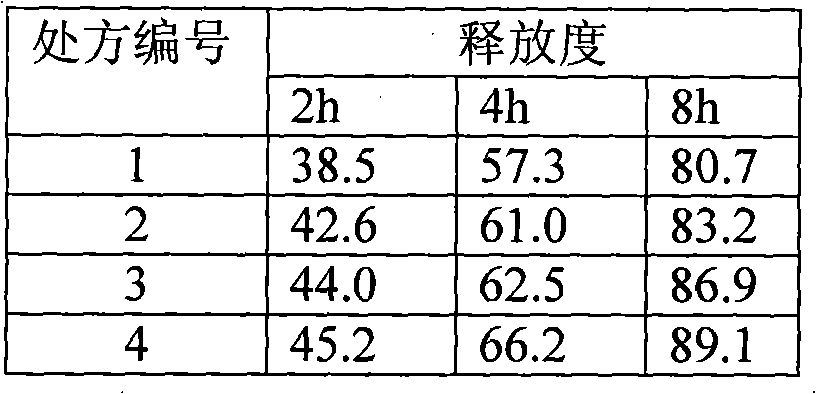
[0082] 1) Prescription composition
[0083] Composition Dosage (1000 tablets)
[0084] Venlafaxine hydrochloride 84.9g
[0085] Hypromellose K4M 70g
[0086] Hypromellose K15M 50g
[0087] Micronized silica gel 50g
[0088] Stearic acid 30g
[0089] 10% PVPK3085% ethanol appropriate amount
[0091] 2) Preparation process:
[0092] (1) Take the prescription amount of venlafaxine hydrochloride, hydroxypropyl methyl cellulose K4M, hydroxypropyl methyl cellulose K15M, micro powder silica gel and stearic acid and mix well;
[0093] (2) 10% PVP K3085% ethanol solution made of soft material, passed through a 20-mesh sieve to make wet granules;
[0094] (3) Dry at 40°C, and pass through a 20-mesh sieve;
[0095] (4) Add the prescribed amount of magnesium stearate and mix well.
[0096] Press the prepared granules in a rotary tablet press to obtain venlafaxine hydrochloride sustained-releas...
Embodiment 2
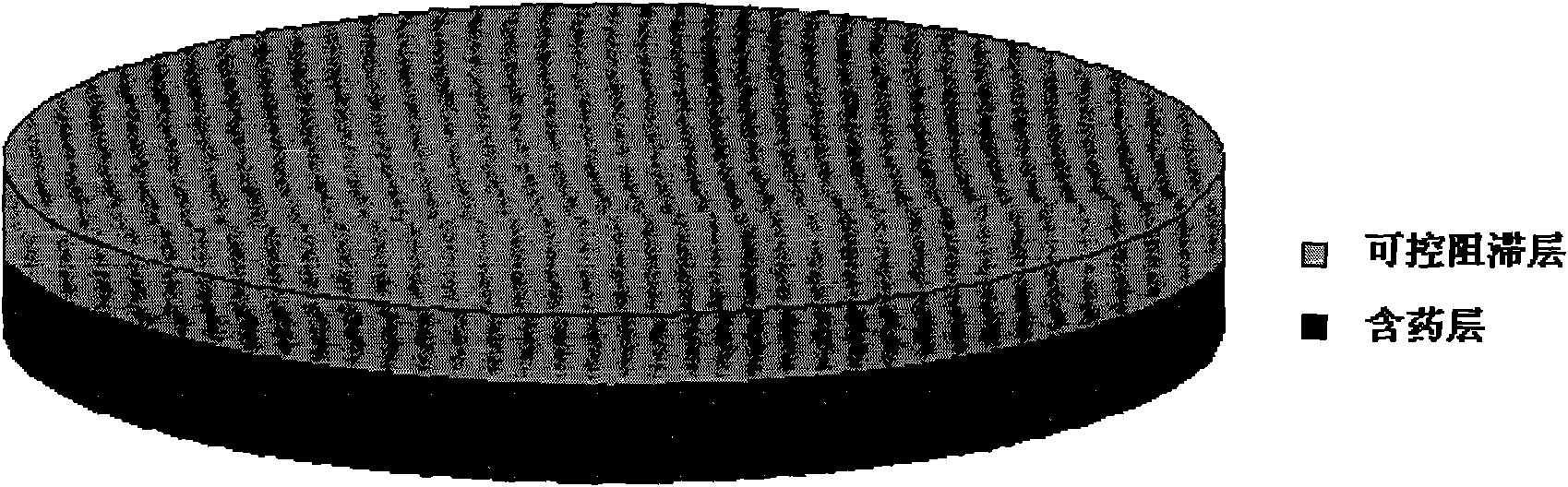
[0102] Example 2 Venlafaxine Hydrochloride Double-layer Sustained-Release Tablets Containing Retention Layer:
[0103] 1) Prescription composition
[0104] Medicinal layer:
[0105] Composition Dosage (1000 tablets)
[0106] Venlafaxine hydrochloride 84.9g
[0107] Hypromellose K4M 70g
[0108] Hypromellose K15M 50g
[0109] Micronized silica gel 50g
[0110] Stearic acid 30g
[0111] 10% PVPK3085% ethanol appropriate amount
[0113] Blocking layer:
[0114] Composition Dosage (1000 tablets)
[0115] Hypromellose K4M 10g
[0116] Hypromellose K100 20g
[0117] Micronized silica gel 15g
[0118] Lactose 35g
[0119] 10% PVPK3085% ethanol appropriate amount
[0120] Magnesium stearate 3g
[0121] 2) Preparation process:
[0122] Medicinal layer:
[0123] (1) Take the prescription amount of venlafaxine hydrochloride, hydroxypropyl methyl cellulose K4M, hydroxypropyl methyl cellulose K15M, micro powder silica gel and stearic acid and mix well;
[0124] (2) 10% PVPK3085% ethano...
Embodiment 3
[0141] Example 3 Venlafaxine Hydrochloride Double-layer Sustained-Release Tablets Containing Retention Layer:
[0142] 1) Prescription composition
[0143] Medicinal layer:
[0144] Composition Dosage (1000 tablets)
[0145] Venlafaxine hydrochloride 84.9g
[0146] Hypromellose K4M 70g
[0147] Hypromellose K15M 50g
[0148] Micronized silica gel 50g
[0149] Stearic acid 30g
[0150] 10% PVPK3085% ethanol appropriate amount
[0151] Magnesium stearate 3g
[0152] Blocking layer:
[0153] Composition Dosage (1000 tablets)
[0154] Hypromellose K4M 30g
[0155] Micronized silica gel 10g
[0156] Stearic acid 10g
[0157] 10% PVPK3085% ethanol appropriate amount
[0158] Magnesium stearate 3g
[0159] 2) Preparation process:
[0160] Medicinal layer:
[0161] (1) Take the prescription amount of venlafaxine hydrochloride, hydroxypropyl methyl cellulose K4M, hydroxypropyl methyl cellulose K15M, micro powder silica gel and stearic acid and mix well;
[0162] (2) 10% PVP K3085% ethanol solution made of soft ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com