A kind of recombinant human fibronectin peptide
A technology of human fibronectin and carrier, which is applied in the field of genetic engineering, can solve the problems of poor processability, excessive molecular weight of fibronectin, and carrying pathogens to harm the human body, etc., to achieve small molecular weight, enhance cell metabolism, and increase cell adhesion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Expression of recombinant human fibronectin peptide in Escherichia coli
[0024] 1 Construct the expression vector for recombinant expression of human fibronectin peptide:
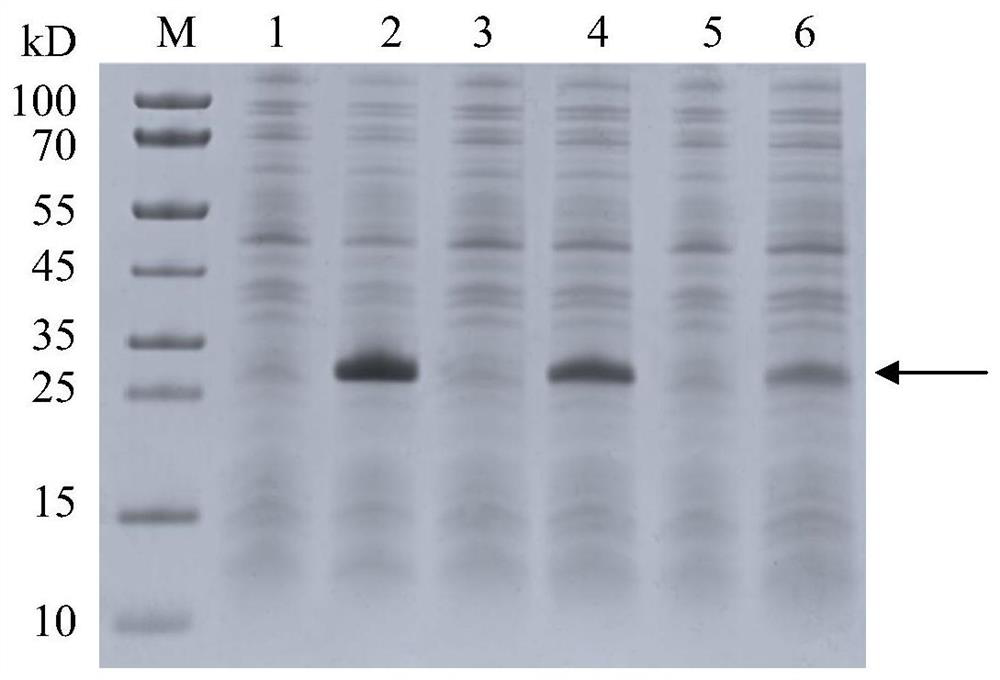
[0025] The DNA fragments (SEQ ID NO.2, SEQ ID NO.3, SEQ ID NO.4) encoding the recombinant human fibronectin peptide were named BL21(DE3) / pET20b- Fnp-1, the expression strain using sequence SEQ ID NO:3 is named BL21(DE3) / pET20b-Fnp-2, and the expression strain using sequence SEQ ID NO:4 is named BL21(DE3) / pET20b-Fnp-3. BL21 (DE3) / pET20b-Fnp-1, BL21(DE3) / pET20b-Fnp-2, BL21(DE3) / pET20b-Fnp-3 induction results are as follows image 3 As shown, the results showed that the expression levels of BL21(DE3) / pET20b-Fnp-1 were higher than those of BL21(DE3) / pET20b-Fnp-2 and BL21(DE3) / pET20b-Fnp-3. The sequence used in subsequent experiments is SEQ ID NO: 2 by default. ) was sent to Jinweizhi Biotechnology Co., Ltd. for whole gene synthesis, and Nde1 and Hind111 (TAKARA Co.Ltd) restriction sites we...
Embodiment 2
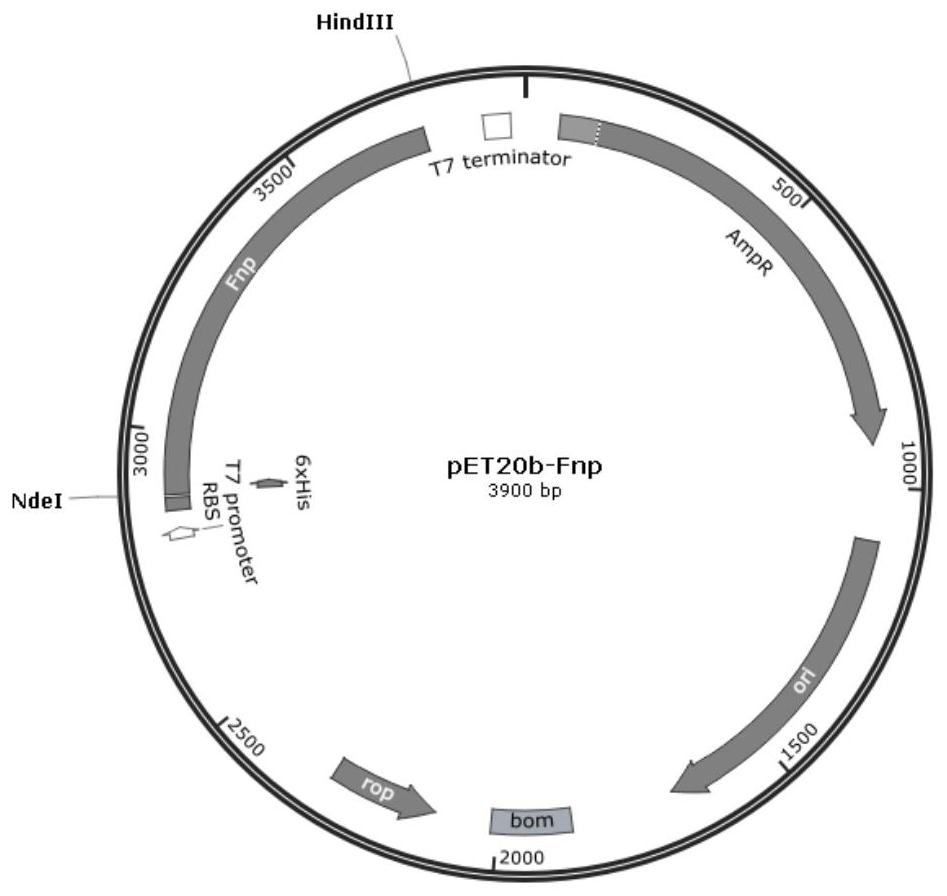
[0029]The gene sequence (SEQ ID NO.2, SEQ ID NO.3 and SEQ ID NO.4) of the artificially synthesized recombinant human fibronectin peptide obtained in Example 1 was introduced into Nde I and Hind 111 at the 5' end and 3' end respectively Restriction site, through Nde I / Hind 111 double digestion, it was connected into pET20b vector, thus obtaining pET20b-Fnp recombinant plasmid. This recombinant plasmid was introduced into competent Escherichia coli BL21 (DE3) for cultivation, and IPTG was used to induce the expression of Fnp protein, and the purified protein was obtained by nickel affinity chromatography, and the molecular weight and immunology of the recombinant protein were analyzed by SDS-PAGE and Western blotting Verify and test its biological activity. Specific steps are as follows:
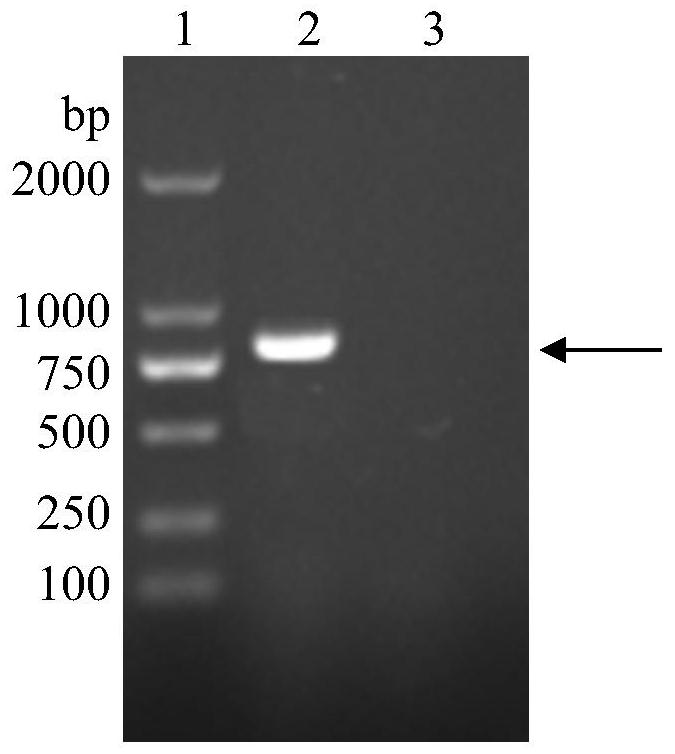
[0030] (1) Construction and identification of pET20b-Fnp recombinant plasmid: the present invention artificially synthesizes the peptide sequence of fibronectin gene, and introduces Nde I and...
Embodiment 3
[0034] (1) Determination of Fnp protein cell adhesion: HaCat cells were cultured with DMEM containing 10% FBS at 37°C, CO 2 Concentration: 5%; wash once with PBS, then add 0.25% trypsin solution for digestion, collect cells by centrifugation; resuspend with DMEM, control the cell density at 6.2×10 4 cells / mL, the cell suspension was inoculated into the culture dish with Fnp protein film on the bottom layer, and the cell density was controlled at 1.5×10 4 individual / mL. Incubate at 37°C for 5h, maintain CO 2 The concentration was 5%; the non-adherent cells were washed away with PBS; counted under a phase-contrast microscope and compared with the number of cells in each group by the MTT method. Positive control group: covered with commercially available bovine fibronectin. see results Figure 5 , the results show that the commercially available bovine fibronectin can increase the adhesion rate of HaCat (ATCC No: CM-1252) cells, and the adhesion of the patented Fnp between 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com