Attenuating bacterial virulence by attenuating bacterial folate transport
A technology of bacteria and folic acid, applied in the direction of antibacterial drugs, bacterial antigen components, antibody medical components, etc., can solve the problem of no cross-protection vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Supplement of embodiment 1 Streptococcus suis bacterial strain S735
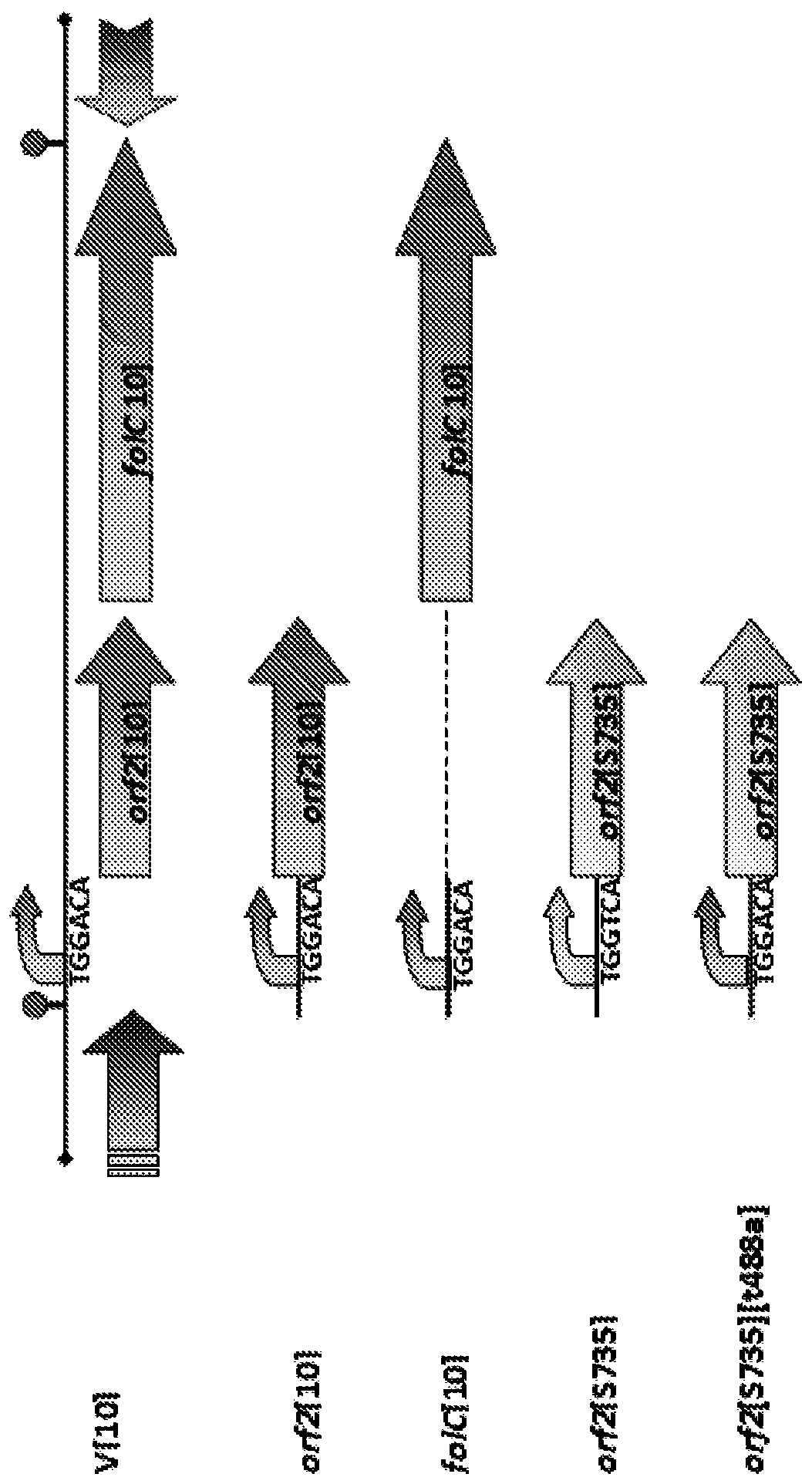
[0036] S735 was supplemented with plasmid pCOM1 containing one of the two ORFs in the V[10] operon (i.e. orf2[10] or folC[10]) previously the putative promoter region of the operon from strain 10, or with Plasmid pCOM1 (orf2[S735]) of orf2 of bacterial strain S735 and homologous upstream promoter ( figure 1 ). To construct these plasmids, primers with restriction sites were designed to amplify the promoter regions of orf2[10] or orf2[S735](comE1–comE2), folC[10](comE4–comE6) or operons ( comE1–comE3) (Table 1). The resulting PCR products orf2[10] and orf2[S735] were digested with restriction enzymes SacI and BamHI, cloned into pKUN19[15], digested with the same restriction enzymes, and subsequently cloned into pCOM1 to obtain pCOM1- orf2[10] and pCOM1-orf2[S735]. The PCR amplicon of folC[10] was digested with restriction enzymes SmaI and BamHI and cloned into pKUN19 cut with the same restriction e...
Embodiment 2
[0037] Example 2 Experimental Infection with Supplementary Isolates
[0038] Experimental infection of sterile piglets obtained by caesarean section was performed as previously described [14]. Prior to infection, piglet sterility was confirmed by plating tonsil swabs on Columbia agar plates containing 6% horse blood. Briefly, 4 or 5 week-old sterile pigs were treated with 10 6 Colony forming units (CFU) of Streptococcus suis for intravenous infection followed immediately by oral administration of 40 mg kg twice daily -1 Body weight of erythromycin (erythromycin-stearate, Abbott B.V., Amstelveen, The Netherlands) to maintain selection pressure on S. suis isolates carrying the pCOM plasmid. Infected pigs were monitored twice daily for clinical signs and tonsil swabs were collected for bacteriological analysis. Euthanize pigs when clinical body weights of arthritis, meningitis, or sepsis are observed after infection with S. suis. Tissue samples of the CNS, serosae and joints ...
Embodiment 3
[0041] Example 3 Experimental infection with strain 10ΔfolT (CBS 140425), experiment 1
[0042] Ten 4-week-old piglets were housed in two groups of five animals at the CVI animal facility. Piglets have free access to feed and fresh water. Animals were provided with warm lights and play materials throughout the experiment. Tonsil swabs from piglets were screened for colonization with S. suis serotype 2 by PCR before the start of the experiment. Only PCR-negative piglets were included in the experiment. After ten days, in the jugular vein with 1,1x10 6 CFU wild type strain 10 or 9.2x10 5 CFU mutant 10ΔfolT infected animals intravenously. The basal temperature of the piglets was monitored daily for a period of three days prior to infection. EDTA blood was collected prior to infection to obtain pre-infection plasma samples, as well as basal levels of white blood cell (WBC) numbers. Infected pigs were monitored for clinical signs three times daily at 8pm, 3am and 9am. Nonsp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com