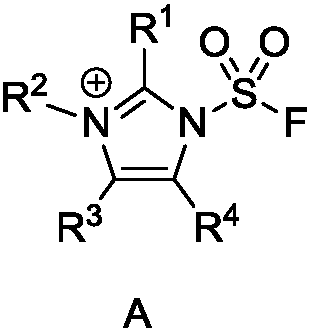
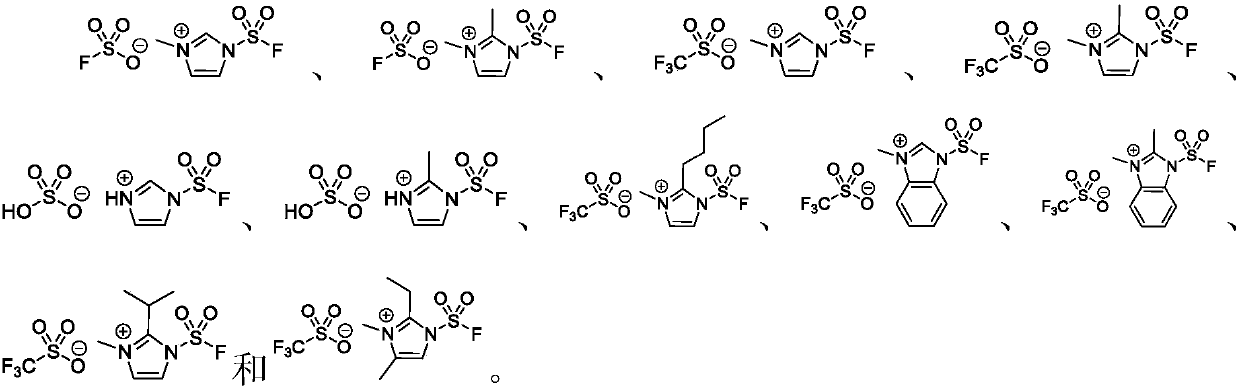
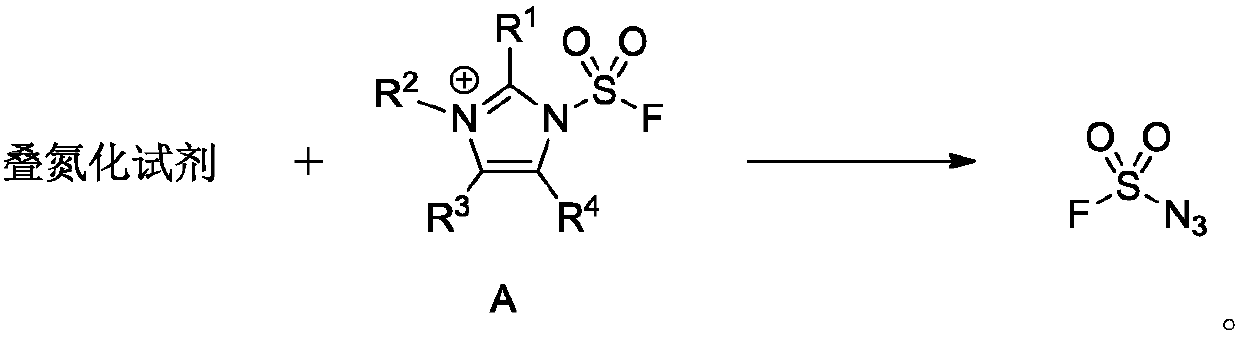
Application of fluorosulfonyl compound to preparation of FSO2N3
A technology of FSO2N3 and fluorosulfonyl, applied in azide acid/azide/halogen azide, organic chemistry, etc., can solve the problems of low safety and poor preparation yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of 1-(fluorosulfonyl)-2,3-dimethyl-1H-imidazole trifluoromethanesulfonate
[0051]
[0052] Add 2-methylimidazole [Compound 1] (49.3g, 600mmol) to the suspension of sodium carbonate (159.1g, 1500mmol) and acetonitrile (600mL) at room temperature, and the reaction system is pumped to negative pressure with a balloon to introduce sulfuryl fluoride Gas [compound 2] (18L, 730mmol), stirred overnight, TLC (petroleum ether: ethyl acetate = 10:1, product R f =0.44) to detect the completion of the reaction, the reaction solution is filtered with silica gel (10-40 mesh), the filter cake is washed with dichloromethane (600mL), the filtrate is extracted with distilled water (3000mL×3), and the aqueous phase is combined with dichloromethane (600mL ) back extraction, the combined organic phases are washed with saturated brine (600mL), dried over anhydrous sodium sulfate, and the filtrate is concentrated by a rotary evaporator (the boiling point of 2-methyl-1H-...
Embodiment 2
[0055] Preparation of 1-(fluorosulfonyl)-3-methyl-1H-imidazole trifluoromethanesulfonate
[0056]
[0057] Add imidazole [compound 5] (1.36g, 20mmol) to sodium carbonate (4.2g, 40mmol) acetonitrile (80mL) suspension at room temperature. ] (0.6L, 25mmol), stirred overnight, TLC (petroleum ether: ethyl acetate=10:1, product R f =0.48) to detect the completion of the reaction, add water (200mL) to the system to separate the phases of the reaction solution, extract with dichloromethane (200mL×3), wash the organic phases with saturated brine (150mL) after merging, dry over anhydrous magnesium sulfate, and Filter through algal earth, and concentrate the filtrate to about 40mL by a rotary evaporator (1H-imidazole-1-sulfonyl fluoride has a low boiling point, the temperature during concentration is controlled below 28°C, and the pressure is controlled above 140torr) to obtain the product 1H-imidazole-1 - a mixed solution of sulfonyl fluoride [compound 6], dichloromethane an...
Embodiment 3
[0060] Preparation of 1-(fluorosulfonyl)-1H-imidazolium bisulfate
[0061]
[0062] Add imidazole [compound 5] (0.68 g, 10 mmol) to sodium carbonate (2.1 g, 20 mmol) acetonitrile (40 mL) suspension at room temperature, and then use a balloon to introduce sulfuryl fluoride gas [compound 2 ] (0.4L, 16mmol), stirred overnight, TLC (petroleum ether: ethyl acetate=10:1, product R f =0.48) to detect the completion of the reaction, add water (100mL) to the system to separate the phases of the reaction solution, extract with dichloromethane (80mL×3), wash with saturated brine (60mL) after the organic phases are combined, dry over anhydrous magnesium sulfate, and Filter through algal earth, and concentrate the filtrate to 20mL by a rotary evaporator (1H-imidazole-1-sulfonyl fluoride has a low boiling point, the temperature during concentration is controlled below 28°C, and the pressure is controlled above 140torr), and the product 1H-imidazole-1-sulfonyl fluoride is obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com