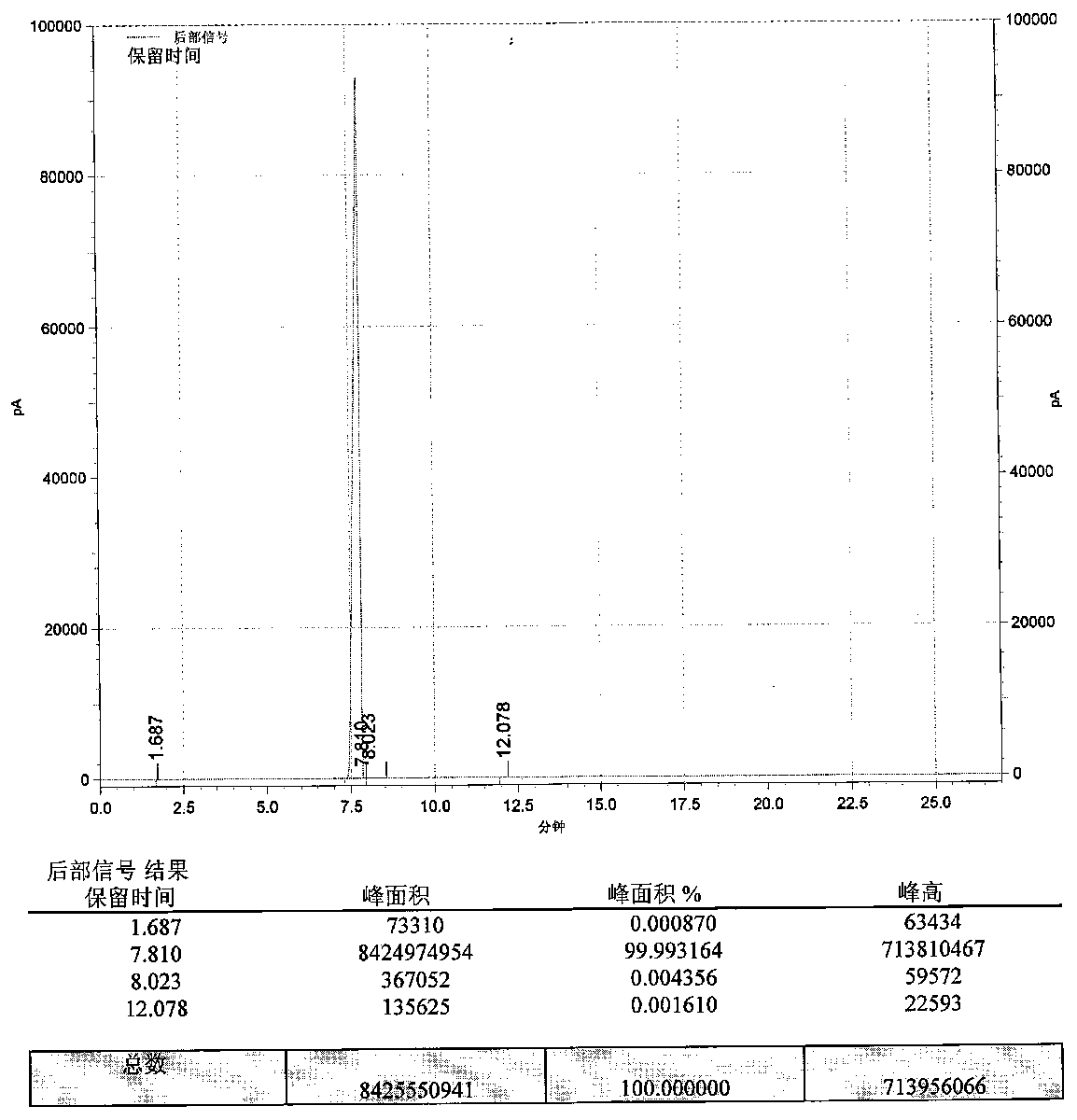
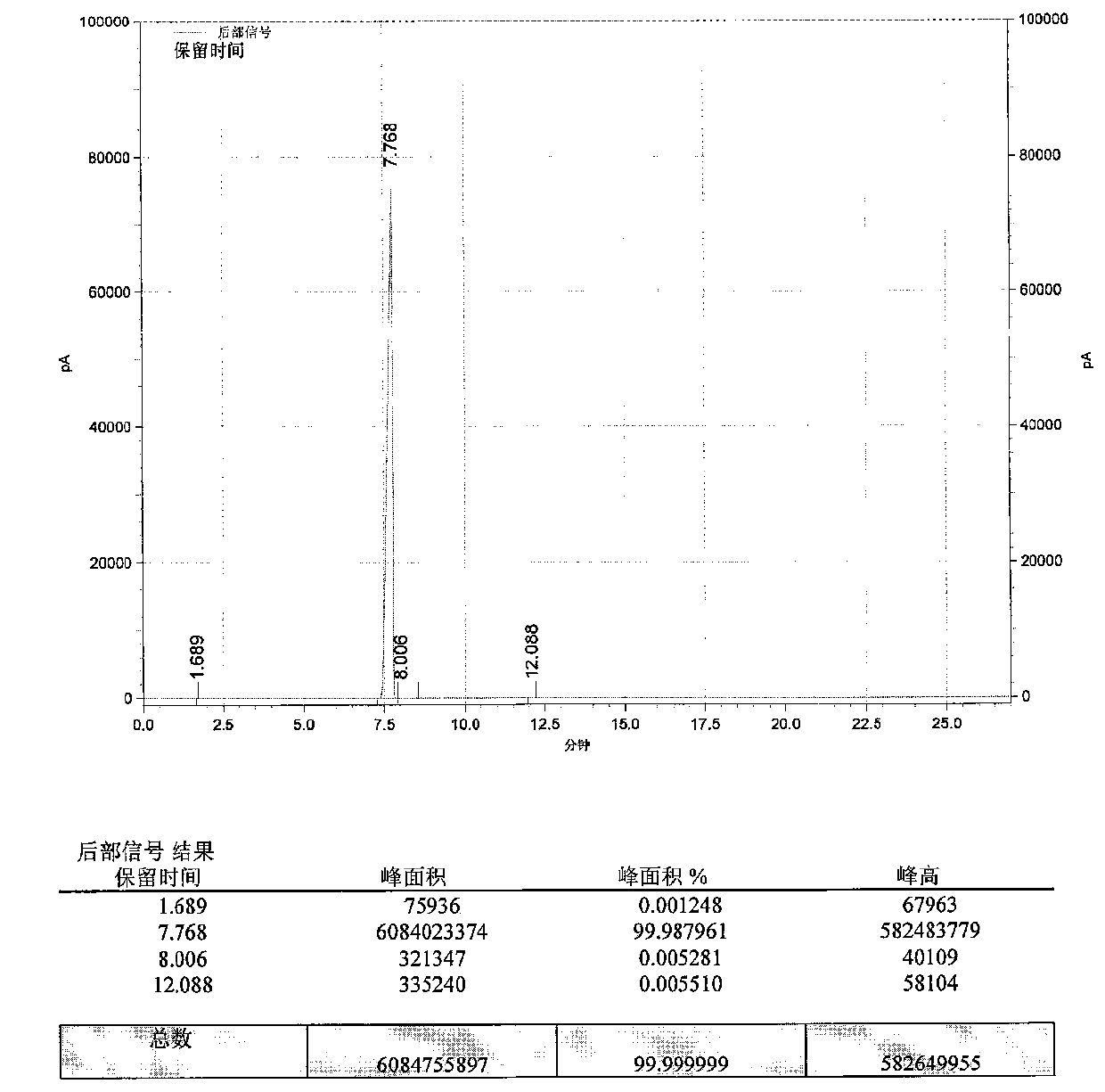
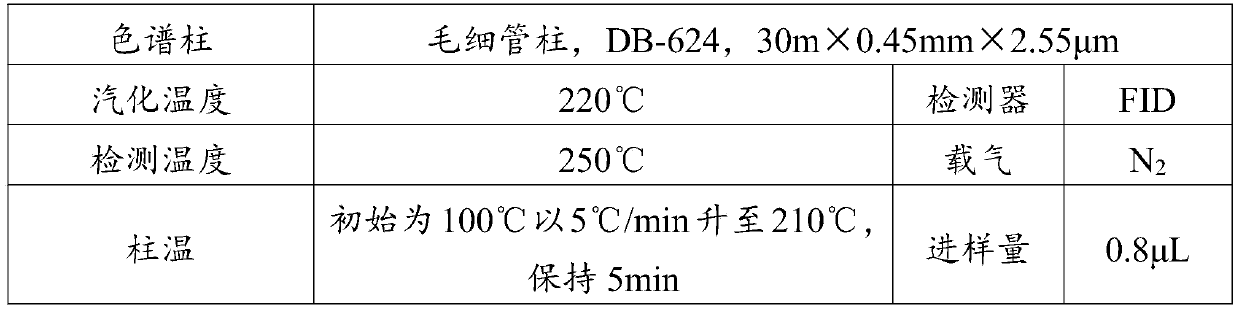
Benzodioxole preparation method
A technology of piperonyl ring and dichloromethane is applied in the field of preparation of piperonyl ring, can solve problems such as low yield of piperonyl ring, environmental pollution, waste of resources and the like, and achieves the effects of reducing production cost, less environmental pollution and easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention provides a kind of preparation method of pepper ring, comprising the following steps:
[0024] Mixing catechol, sodium hydroxide, water and simethicone to carry out the first reaction to obtain a sodium catechol solution;
[0025] The sodium catecholate solution, tetrabutylammonium chloride and dichloromethane are mixed for condensation reaction to obtain the piperonyl ring.
[0026] The invention mixes catechol, sodium hydroxide, water and simethicone oil to carry out the first reaction to obtain the sodium catechol solution.
[0027] In the present invention, the weight ratio of catechol, sodium hydroxide, water and simethicone oil is preferably 1:0.76~0.8:1.4~1.5:0.42~0.57, more preferably 1:0.77~0.79: 1.4 to 1.5:0.45 to 0.55, more preferably 1:0.78:1.45:0.5.
[0028] In the present invention, the mixing of said catechol, sodium hydroxide, water and simethicone is preferably carried out in a phenol resolving kettle; in combination with a phenol resolv...
Embodiment 1
[0057] Put 350kg of catechol, 266kg of sodium hydroxide, and 490kg of water into a phenol-dissolving kettle equipped with 150kg of dimethyl silicone oil, stir and dissolve, maintain 40°C, stop stirring for later use, and obtain sodium catechol solution; Put 750kg of methylene chloride and 10.5kg of tetrabutylammonium chloride into the autoclave to obtain tetrabutylammonium chloride methylene chloride solution, raise its temperature to 60°C, and inject the sodium catecholate solution into the In the autoclave, use it for 10 hours, after feeding, keep the temperature at 60-70°C for 2h, after the reaction, cool the condensation reaction solution to 20°C, add 3.5kg of activated carbon into the autoclave, stir for 0.5h, press filter, The filter cake is washed with dichloromethane, the filtrate and the washing liquid are combined, and the liquid is separated to obtain the first water phase and the first oil phase; the first water phase is washed once with 75kg of dichloromethane, and...
Embodiment 2
[0063] Put 350kg of catechol, 280kg of sodium hydroxide and 525kg of water into a phenol-dissolving kettle equipped with 200kg of dimethyl silicone oil, stir and dissolve, maintain 60°C, stop stirring for later use, and obtain sodium catechol solution; Put 900kg of dichloromethane and 17.5kg of tetrabutylammonium chloride into the autoclave, raise the temperature to 70°C, inject the sodium catechol solution into the autoclave with a plunger pump, and take 12 hours. Insulate at 70°C for 2h, cool the obtained condensation reaction solution to 30°C, add 3.5kg of activated carbon into the autoclave, stir for 0.5h, press filter, wash the filter cake with dichloromethane, combine the filtrate and washing liquid, and separate the liquids to obtain The first water phase and the first oil phase; the first water phase is washed once with 75kg of dichloromethane, and the combined dichloromethane washing solution and the oil phase are used as the second oil phase, and the first water phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com