Nickel-doped cobalt selenide electrocatalytic hydrogen evolution catalyst and preparation method thereof
A cobalt selenide electrocatalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, electrodes, etc., can solve the problems of complex preparation steps, high cost, poor safety, etc., and achieve simple preparation steps, low cost, The effect of cost security
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
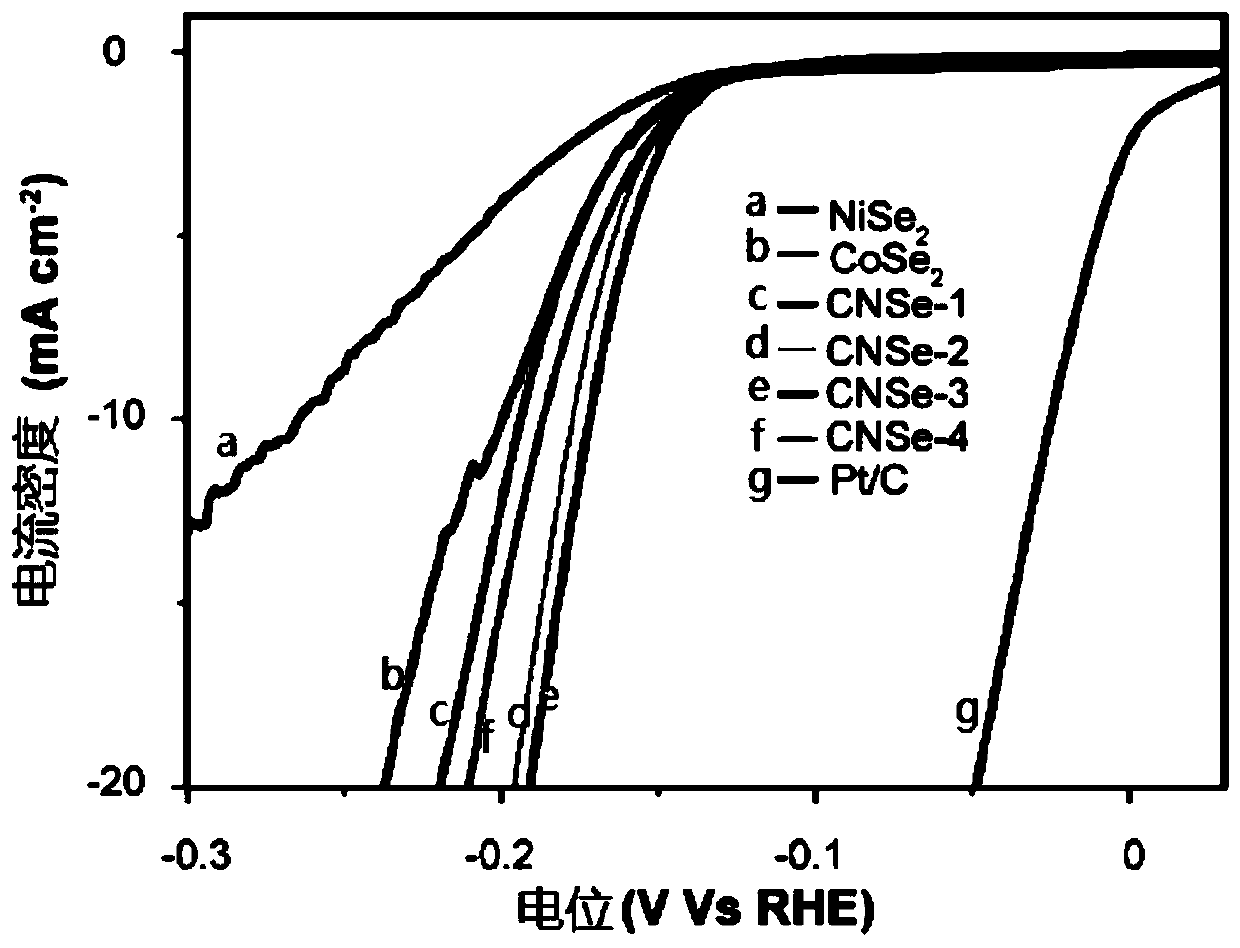
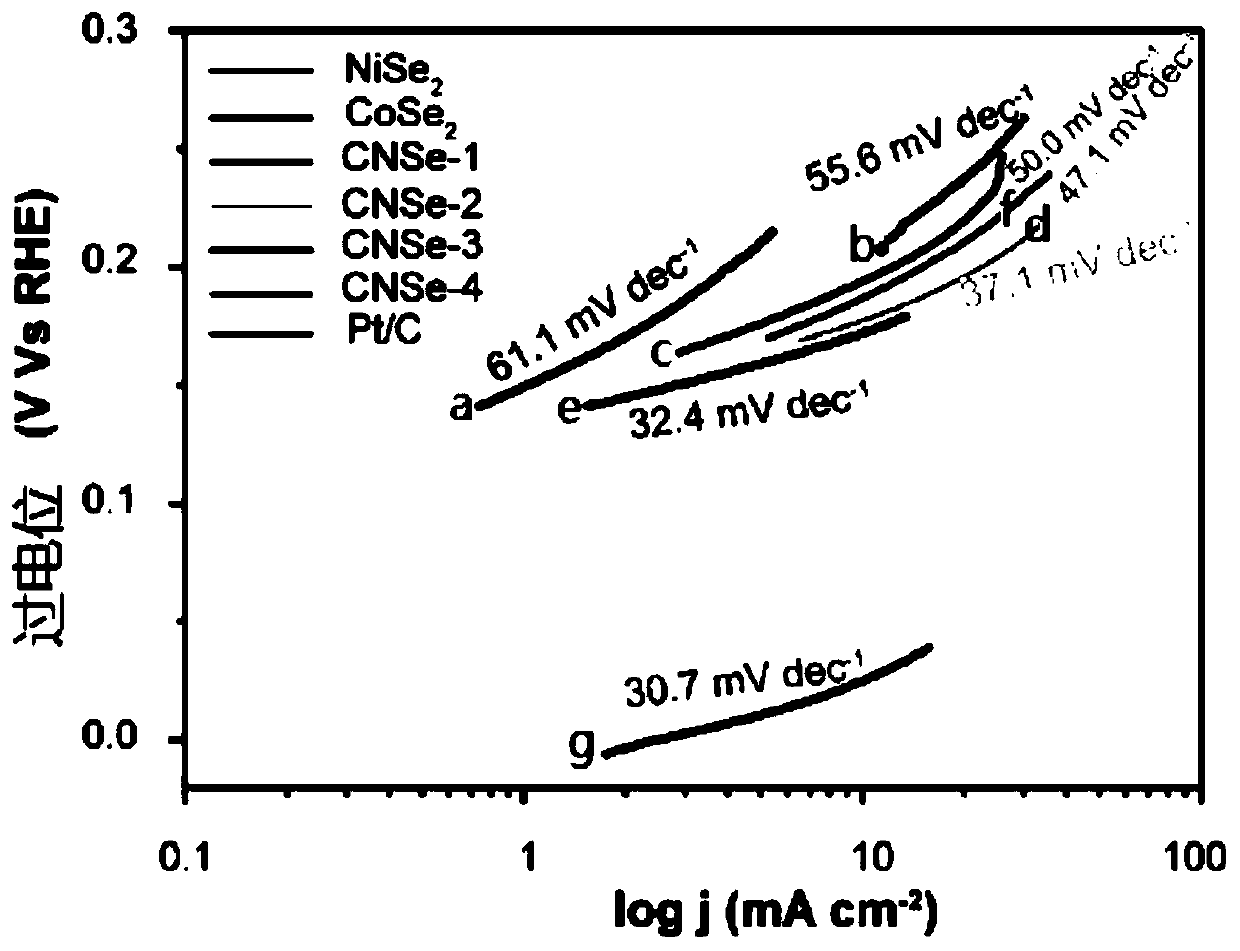
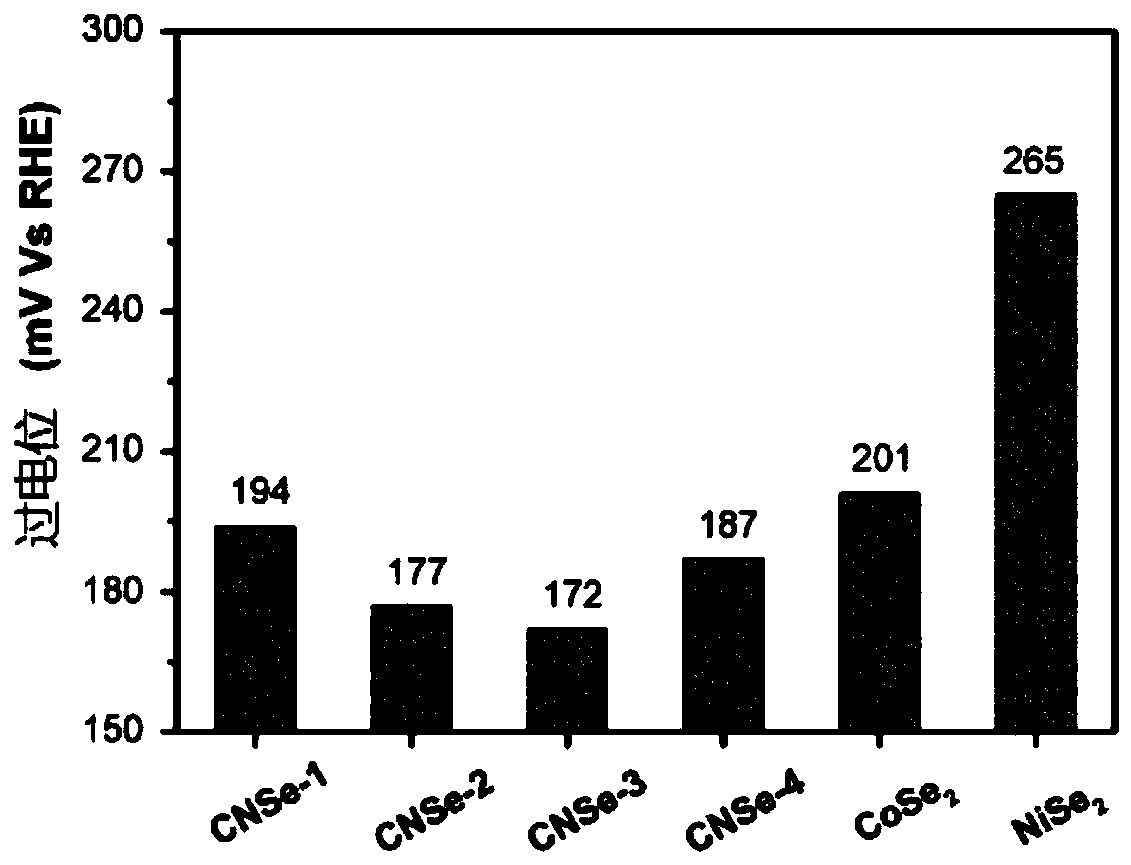
[0021] Specific embodiment 1: The nickel-doped cobalt selenide electrocatalytic hydrogen evolution catalyst of this embodiment has the general chemical formula of Co x Ni y Se 2 , X = 0.6 ~ 0.8, y = 0.1 ~ 0.35.
specific Embodiment approach 2
[0022] Specific embodiment two: The preparation method of nickel-doped cobalt selenide electrocatalytic hydrogen evolution catalyst described in specific embodiment one is carried out according to the following steps:
[0023] 1. Press Co x Ni y Se 2 The stoichiometric ratio of selenium powder, Co(NO 3 ) 2 ·6H 2 O and Ni (NO 3 ) 2 ·6H 2 O, Co x Ni y Se 2 Where x = 0.6 ~ 0.8, y = 0.1 ~ 0.35; then weigh potassium hydroxide solution, EDTA-2Na and ultrapure water;
[0024] 2. Dissolve selenium powder in potassium hydroxide solution, stir for 25~35min, add Co(NO 3 ) 2 ·6H 2 O, Ni(NO 3 ) 2 ·6H 2 O, EDTA-2Na and ultrapure water, continue to stir for about 1~1.5h to obtain a mixed solution;
[0025] 3. Transfer the mixture to a Teflon reaction kettle, react for 10-16h at a temperature of 160-200℃, and cool to room temperature naturally;
[0026] 4. The black precipitate obtained by the reaction is washed with water and ethanol in turn, and then placed in a vacuum oven at 60-65°C for 12-15 hour...
specific Embodiment approach 3
[0027] Specific embodiment three: This embodiment is different from the specific embodiment two in that the concentration of the potassium hydroxide solution described in step one is 20-25 mol / L; the other is the same as the specific embodiment two.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com