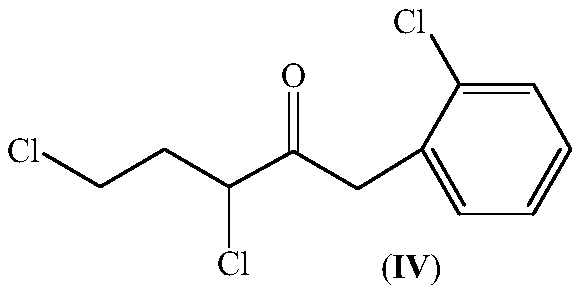
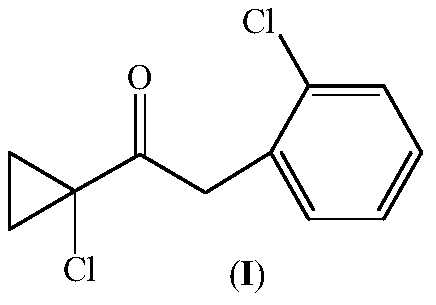
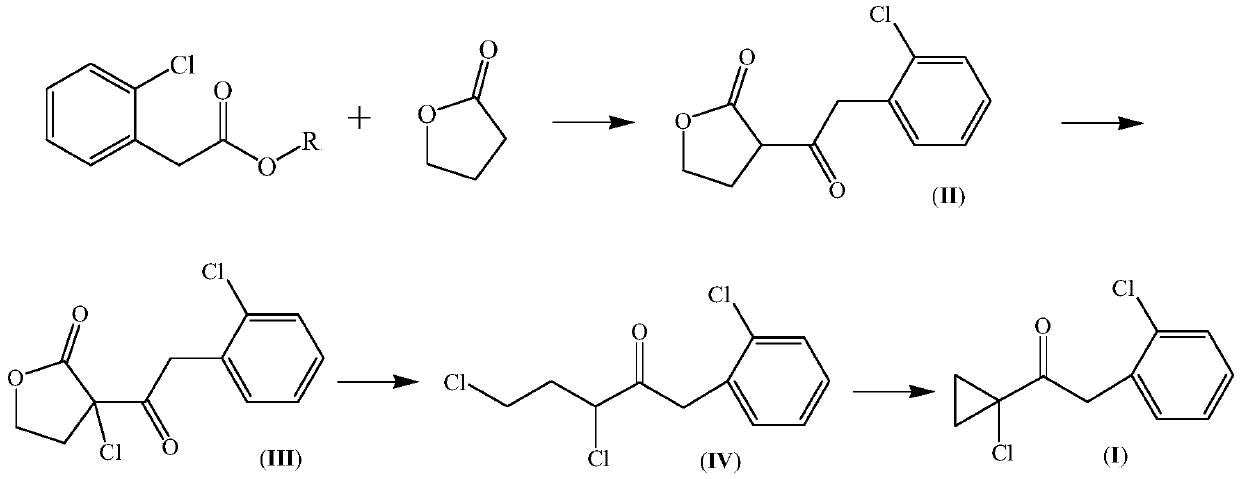
Preparation method of 1-(1-chlorocyclopropyl)-2-(2-chlorphenyl)ethanone and intermediate thereof
A chlorocyclopropyl, chlorophenyl technology, applied in the field of preparation of 1--2-ethanone, can solve the problems of high cost, restricting industrial development, harsh reaction conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Under the protection of nitrogen, 27.0g of sodium metal was added to 300mL of toluene, heated to 100°C to 110°C and stirred for 30 minutes; 185.0g of methyl 2-chlorophenylacetate and 90.0g of γ-butyl were added dropwise to the above suspension system A mixture of lactones; after the addition, continue to stir and react for 3 hours, during which the formed low boilers are evaporated from the reaction system; Stir for 30 minutes; filter with suction, wash the filter cake with 50mL of toluene, and collect the filtrate; wash the obtained filtrate with 200mL×2 water, and precipitate under reduced pressure to obtain 3-[2-(2-chlorophenyl)acetyl]-4,5 - 229.0 g of dihydrofuran-2(3H)one (II), which was in a yellow semi-solid state after standing, with a content of 93.3% by chromatographic analysis.
[0048] The above crude product was subjected to column chromatography (ethyl acetate / n-hexane 1:1) to obtain an off-white solid with a chromatographic content of 99.1%: EIMS (m / z): 2...
Embodiment 2
[0050] With reference to Example 1, 50.0 g of sodium amide was used to replace the sodium metal therein to obtain 3-[2-(2-chlorophenyl) acetyl]-4,5-dihydrofuran-2(3H) ketone (II) 225.0 g, chromatographic analysis content 90.8%.
Embodiment 3
[0052] At a temperature of 20-30°C, 30.0g of γ-butyrolactone was added dropwise to a mixture consisting of 60.0g of ethyl 2-chlorophenylacetate, 25.0g of sodium methoxide and 100mL of tetrahydrofuran, and stirring was continued for 1 hour; the obtained reaction mixture Gradually heat up to reflux state and keep reacting for 4 hours; cool to room temperature, add hydrochloric acid solution to the reaction system dropwise to about pH4, then add 150mL dichloromethane and 200mL water, continue to stir for 30 minutes and then let stand to separate; the water layer Add 50 mL of dichloromethane for extraction, combine the organic phases and wash with 100 mL of water, and desolvate under reduced pressure to obtain 70.0 g of an orange viscous residue, namely 3-[2-(2-chlorophenyl)acetyl]-4,5- Dihydrofuran-2(3H)one (II) crude product, the content of chromatographic analysis is 87.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com