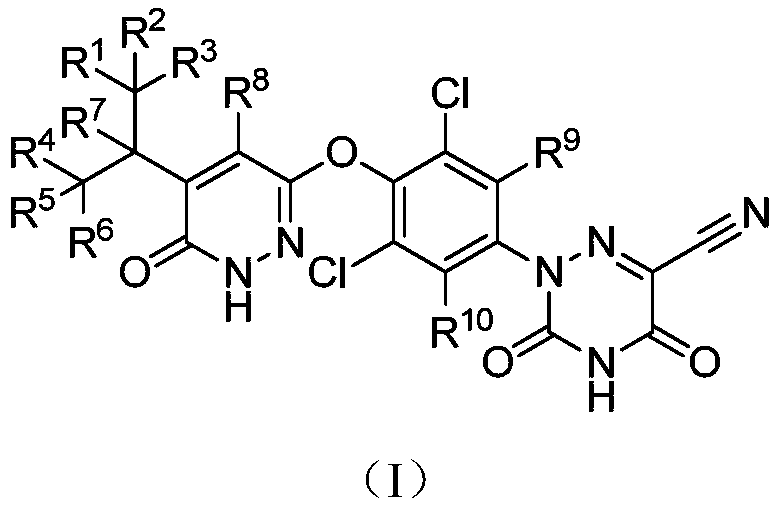
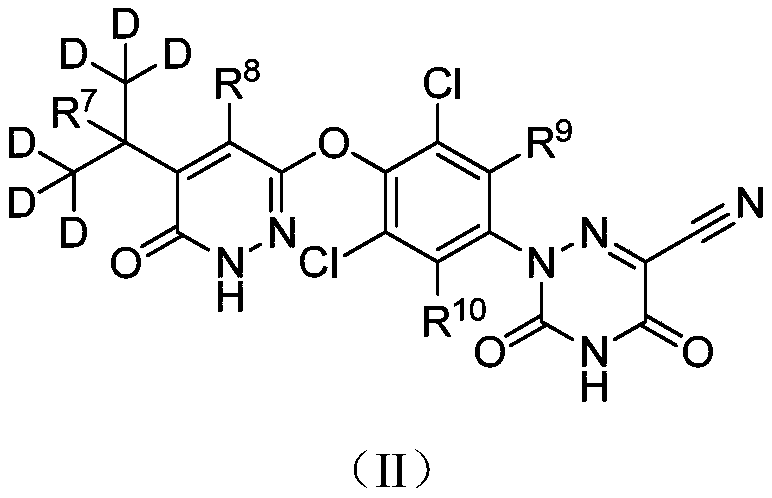
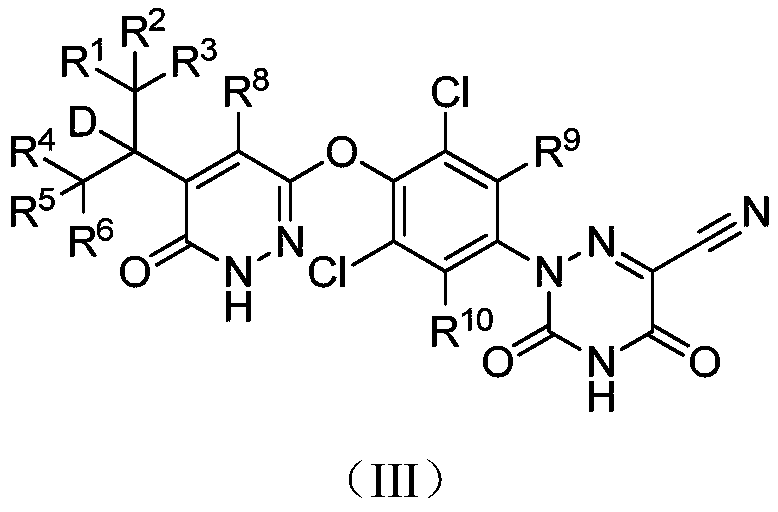
Deuterated MGL-3196 compound and application thereof
A technology for compounds and uses, applied in the directions of active ingredients of heterocyclic compounds, metabolic diseases, drug combinations, etc., can solve problems such as deteriorating pharmacokinetic properties, and achieve good pharmacokinetic properties, treatment of dyslipidemia, and low clearance rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. Synthesis of 2-(3,5-dichloro-4-((5-(1,1,1,3,3,3-hexadeuteriopropan-2-yl)-6-oxo-1, 6-dihydropyridazin-3-yl)oxy)benzene)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile (1)
[0040]
[0041] 2-trideuteromethyl-3,3,3-trideuteropropionic acid A was prepared by literature method (Canadian Journal of Chemistry, 2014, 92, 305).
[0042]
[0043] Measure 350mL of ethanol into a 500mL three-necked round bottom flask, and stir at room temperature. Subsequently, Na flakes (9.9 g, 430.79 mmol) were slowly added in batches to the system, and when the system was completely clear, the system was transferred to an oil bath to continue heating and stirring. When the internal temperature of the system rose to 70°C, diethyl malonate (30 g, 187.30 mmol) was added dropwise to the system, and after the dropping was completed, the mixture was kept stirring for 15 minutes. Deuteromethyl iodide (57 g, 393.33 mmol) was added dropwise to the system, and the rate of a...
Embodiment 2
[0056] Example 2. Synthesis of 2-(3,5-dichloro-4-((5-(heptadeuterioisopropylpropyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy)) Phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile (2)
[0057]
[0058] Synthesis of 2,3,3,3-tetradeutero-2-(trideuteromethyl)propionic acid (compound B):
[0059]Weigh 2,2-bis(trideuteromethyl)malonic acid (3g, 34mmol) into a 100mL single-neck round bottom flask, add heavy water (15mL) to it, and place the system in a water bath at 60°C The solvent was removed by rotary evaporation, and the operation was repeated twice. Transfer the above substrate into a 35 mL sealed tube, add heavy water (9 mL), seal and place in an oil bath at 160° C., and stir the reaction overnight. After 12 hours, the heating was stopped, and the system was allowed to cool to room temperature, and the solvent was removed at low temperature to obtain compound B (2.1 g) as a colorless transparent oily liquid. It was directly used in the next reaction without furth...
Embodiment 3
[0066] Example 3. Synthesis of 2-(3,5-dichloro-4-((5-(1,1,1-trideuteroprop-2-yl)-6-oxo-1,6-dihydropyridazine -3-yl)oxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile (3)
[0067]
[0068] Synthesis of 2-(trideuteromethyl)propionic acid (Compound C)
[0069]
[0070] (1) Synthesis of compound diethyl-2-deuteromethyl-2-methylmalonate
[0071] Measure 350mL of ethanol into a 500mL three-necked round bottom flask, and stir at room temperature. Subsequently, Na flakes (9.9 g, 430.79 mmol) were slowly added in batches to the system, and when the system was completely clear, the system was transferred to an oil bath to continue heating and stirring. When the internal temperature of the system rose to 70°C, 2-methyl-diethyl malonate (20.0 g, 114.80 mmol) was added dropwise to the system, and after the dropping was completed, the mixture was kept stirring for 15 minutes. Deuteromethyl iodide (18.5 g, 196.6 mmol) was added dropwise to the system, and the rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com