Biomarkers and detection kits for prostate cancer diagnostic grading and benign and malignant prediction
A technology of biomarkers and diagnostic kits, which is applied in the fields of biotechnology and medicine, can solve problems such as general specificity, specificity and sensitivity are not very ideal, achieve broad application prospects, save manpower and material costs, and improve clinical The effect of applying value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1 Separation of Urine Exosomes
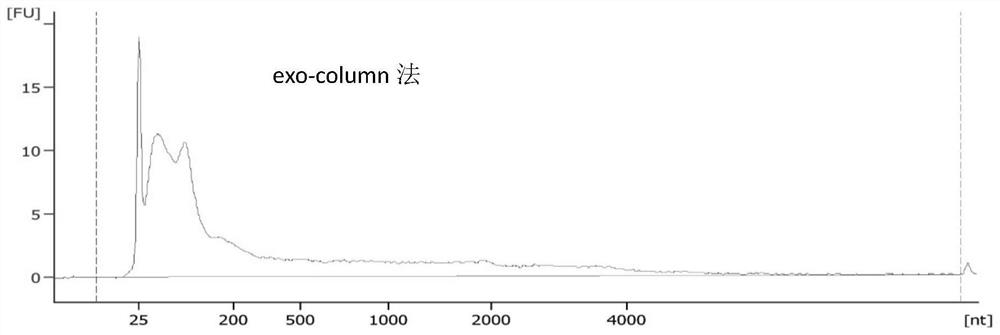
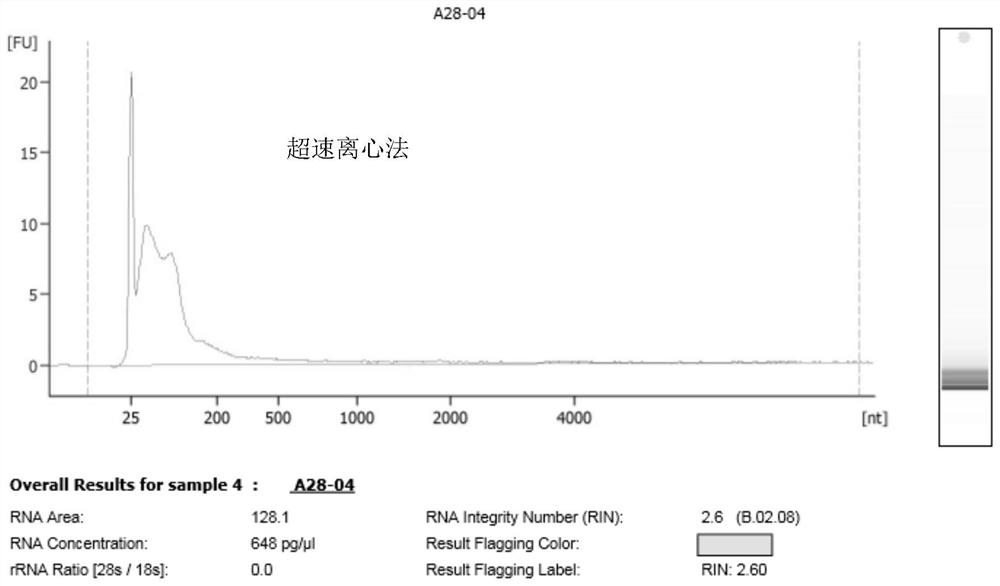
[0105] 1. UC method: 40ml human morning urine sample, centrifuged at low speed to remove cells, centrifuged at 17000g for 30min to remove cell debris and apoptotic bodies, etc., ultracentrifuged at 100000g for 2h, removed supernatant, resuspended in PBS and centrifuged again at 100000g for 2h, resuspended in 100ul PBS Exosomes were obtained by suspension pellet.
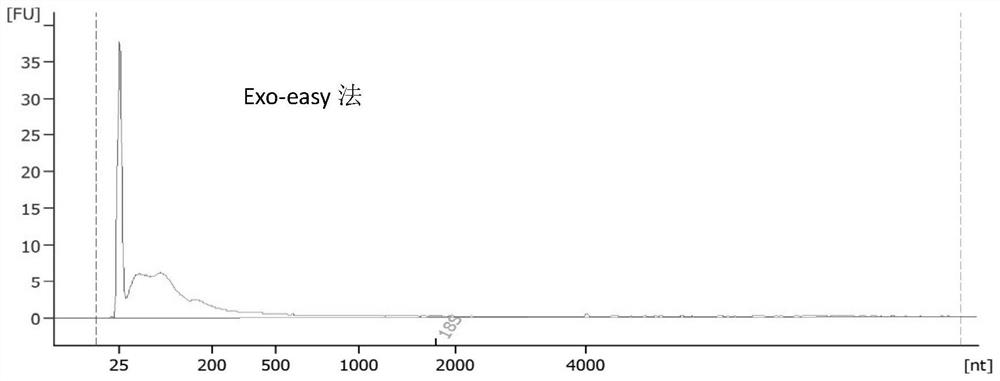
[0106] 2. Ultrafiltration method: After the sample is thawed, centrifuge at 17000xg for 30min; after centrifugation, the sample passes through 0.45um and 0.22um in turn; prepare two ultrafiltration centrifuge tubes, and take 15ml of the sample filtered by the filter membrane in each ultracentrifuge tube, 4500xg at room temperature Centrifuge for 15 minutes; carefully take out the centrifuge tube, discard the liquid at the bottom of the ultrafiltration tube, then resuspend the retentate with 5ml of the remaining filtered urine and 10ml of 1XPBS, invert 3-4 times to mix t...
Embodiment 2
[0111] Example 2 Screening of differentially expressed genes
[0112] In order to screen the urinary exosome markers related to prostate cancer patients (including PSA>20ng / ml and 4-20ng / ml prostate cancer patients) and controls (normal people and interfering samples such as prostatitis and prostatic hypertrophy) In 20 cases, no less than 30ml of morning urine was taken, and the exosomes in the urine were separated by classical ultracentrifugation method and RNA was extracted. The obtained RNA was subjected to miRNA and long RNA library sequencing (including mRNA, LncRNA and CircRNA). The obtained data was analyzed by bioinformatics, and the differentially expressed RNAs in prostate cancer patients and controls were compared, and the genes with differential expression folds of more than 4 times were selected and miRNA sequencing and transcriptome sequencing data organized in the TCGA (The Cancer Genome Atlas) database Correlation analysis was carried out to obtain differential...
Embodiment 3
[0115] Example 3 Validation of selected gene markers by real-time fluorescent PCR
[0116] 1. Design and synthesis of primer probes
[0117] Primer Premier 5 software and Primer-BLAST (NCBI) were used to design primers and probes for 8 prostate cancer markers listed in Table 1 and 3 internal reference genes, and the sequences are shown in Table 2. The design principles are as follows: 1) the amplified fragment is less than 150bp; 2) at least one primer spans the exon-exon boundary; 3) the Tm value of the probe is at least 5 degrees higher than that of the primer. After the design is completed, it will be handed over to a qualified company for synthesis, in which the 5' end of the probe is labeled with the FAM group, and the 3' end is labeled with BHQ1.
[0118] Table 2 Prostate cancer markers of the present invention and primer probe sequences of internal reference genes
[0119]
[0120]
[0121] *marked as internal reference genes.
[0122] 2. Reverse transcription ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com