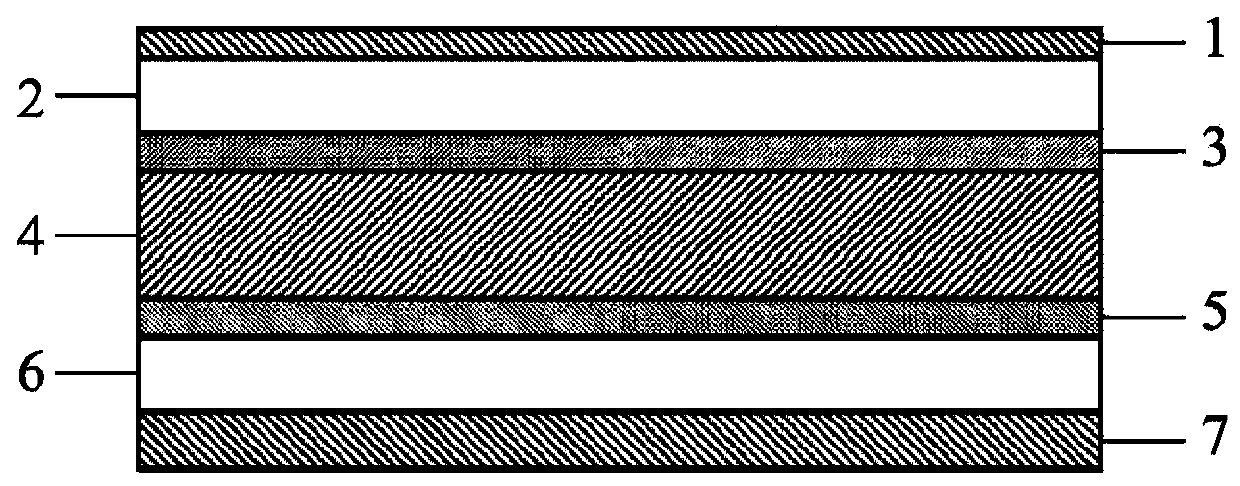
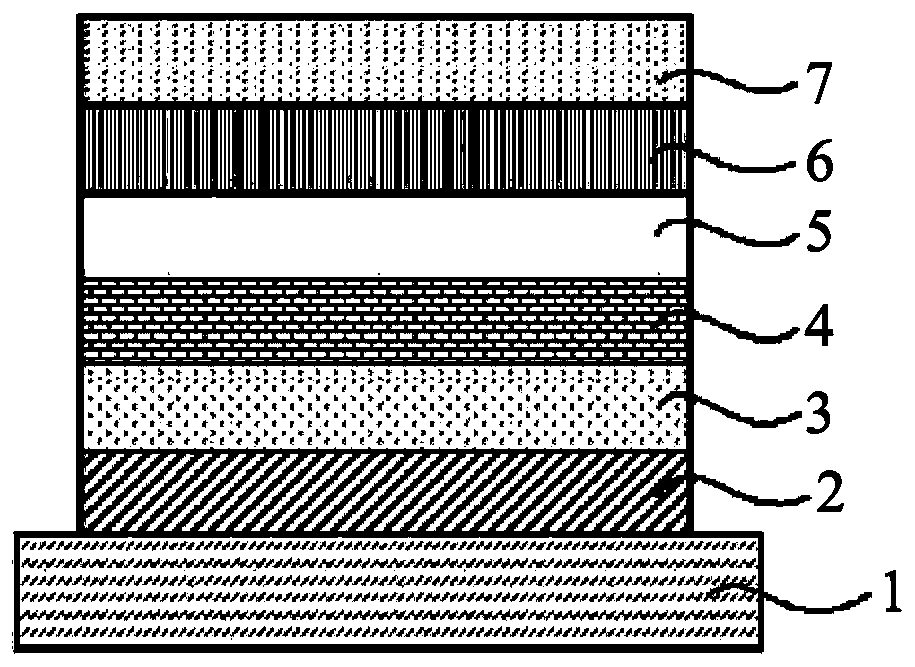
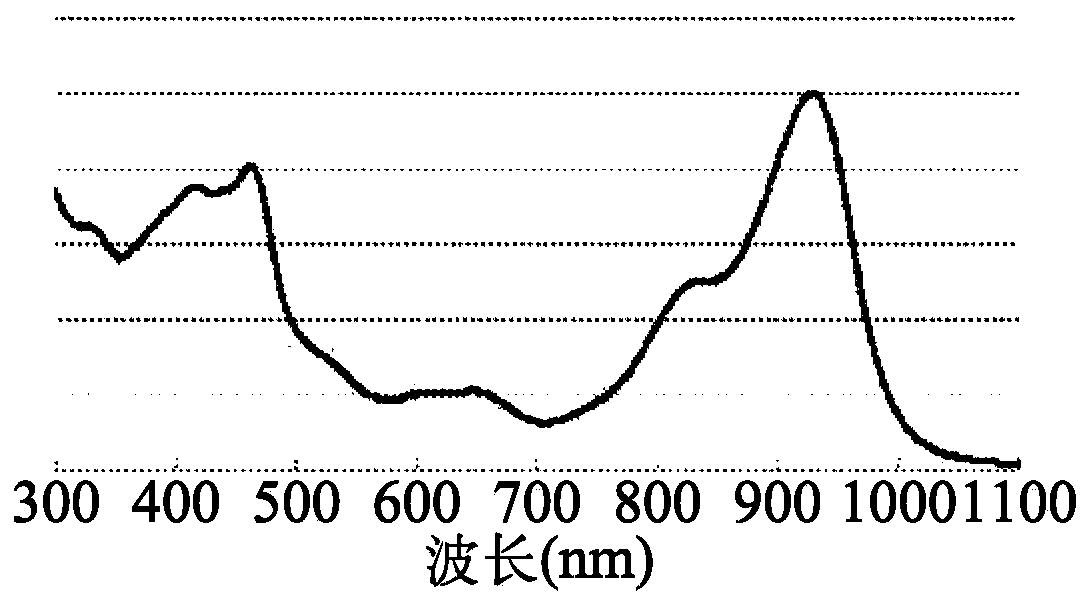
Dibenzopyrromethene boron chelate compound, near-infrared light-absorbing material, thin-film, and organic electronic device
A compound and hydrogen atom technology, applied in the field of dibenzopyrromethine boron chelate compounds, can solve the problems of unmentioned photoelectric conversion characteristics, poor photoelectric conversion characteristics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0193] Embodiment 1 (synthesis of compound of the present invention)
[0194] The compound of the present invention represented by formula (1-22) is synthesized by the following scheme.
[0195]
[0196] (Step 1) In the described scheme, the synthesis of the intermediate compound represented by formula (2-2)
[0197] In a flask, (4-acetyl-3-biphenyl) (3-methoxy-2-thienyl) ketone (in the scheme, the compound represented by formula (2-1)) (68.3 mmol) Dissolve in ethanol (700mL) and acetic acid (150mL), add ammonium acetate (400mmol) and ammonium chloride (70mmol) after heating to 65°C, heat up to 90°C and stir for 3 hours. The reaction liquid was air-cooled, neutralized with a saturated hydrocarbon sodium aqueous solution, and the generated solid was collected by filtration to obtain an intermediate compound represented by formula (2-2) (31.4 mmol, yield 46% by mass).
[0198] The intermediate compound represented by formula (2-2) 1 The measurement results of H NMR are as ...
Embodiment 2
[0208] Embodiment 2 (synthesis of compound of the present invention)
[0209] (Step 4) Synthesis of the compound of the present invention represented by the formula (1-39) of the specific example
[0210] In addition to using (4-acetyl-3-biphenyl)(5-(2,1,3-benzothiadiazol-5-yl)-3-methoxy-2-thienyl)methanone substitution formula Except for the compound represented by (2-1), the compound of the present invention represented by formula (1-39) was obtained by following steps 1 to 3 of Example 1. (0.65mmol, yield 43% by mass)
[0211] The measurement results of the molecular weight of the compound represented by the formula (1-39) are as follows.
[0212] EI-MS (m / z): 868[M] +
Embodiment 3
[0213] Embodiment 3 (synthesis of compound of the present invention)
[0214] (Step 5) Synthesis of the compound of the present invention represented by the formula (1-48) of the specific example
[0215] In addition to using (3-acetyl-4-biphenyl)(5-(2,1,3-benzothiadiazol-5-yl)-3-methoxy-2-thienyl)methanone substitution formula Except for the compound represented by (2-1), the compound of the present invention represented by the formula (1-48) was obtained by following steps 1 to 3 of Example 1. (1.3 mmol, yield 84% by mass).
[0216] The measurement results of the molecular weight of the compound represented by the formula (1-48) are as follows.
[0217] EI-MS (m / z): 868[M] +
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron work function | aaaaa | aaaaa |
| coating thickness | aaaaa | aaaaa |
| coating thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com