Process for continuously producing an aminobenzoate derivative, and synthesis system thereof
A technology of aminobenzoate and nitrobenzoic acid, which is applied in the field of continuous production of aminobenzoate derivatives and its synthesis system, which can solve the problems of unstable product quality, high labor intensity, and high labor cost , to achieve the effects of low conversion rate of esterification reaction, high production efficiency and low labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Reaction raw materials: ethanol (commercially available industrial ethanol, content 99%); p-nitrobenzoic acid (commercially available, content 98%); hydrogen (commercially available, content 99%).
[0049] Esterification catalyst: industrial concentrated sulfuric acid (commercially available, content 98%) or p-toluenesulfonic acid (commercially available, content 99%);
[0050] Catalytic hydrogenation reaction catalyst: commercially available palladium carbon containing 1-5% palladium; dosage is 0.5%-1% of p-nitrobenzoic acid.
[0051] Solvent: the solvent of esterification reaction and catalytic hydrogenation reaction is ethanol (commercially available industrial ethanol, content 99%);
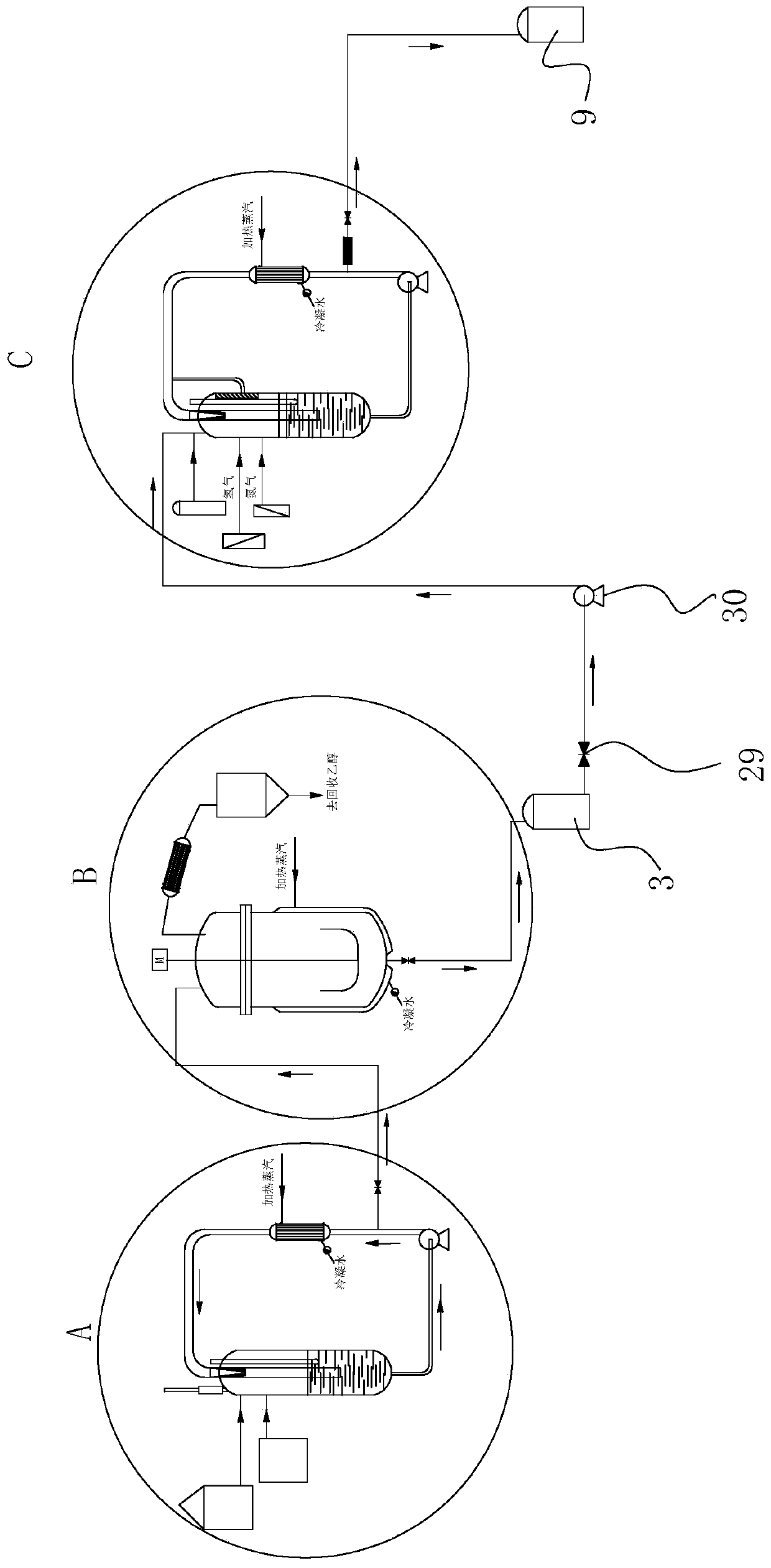
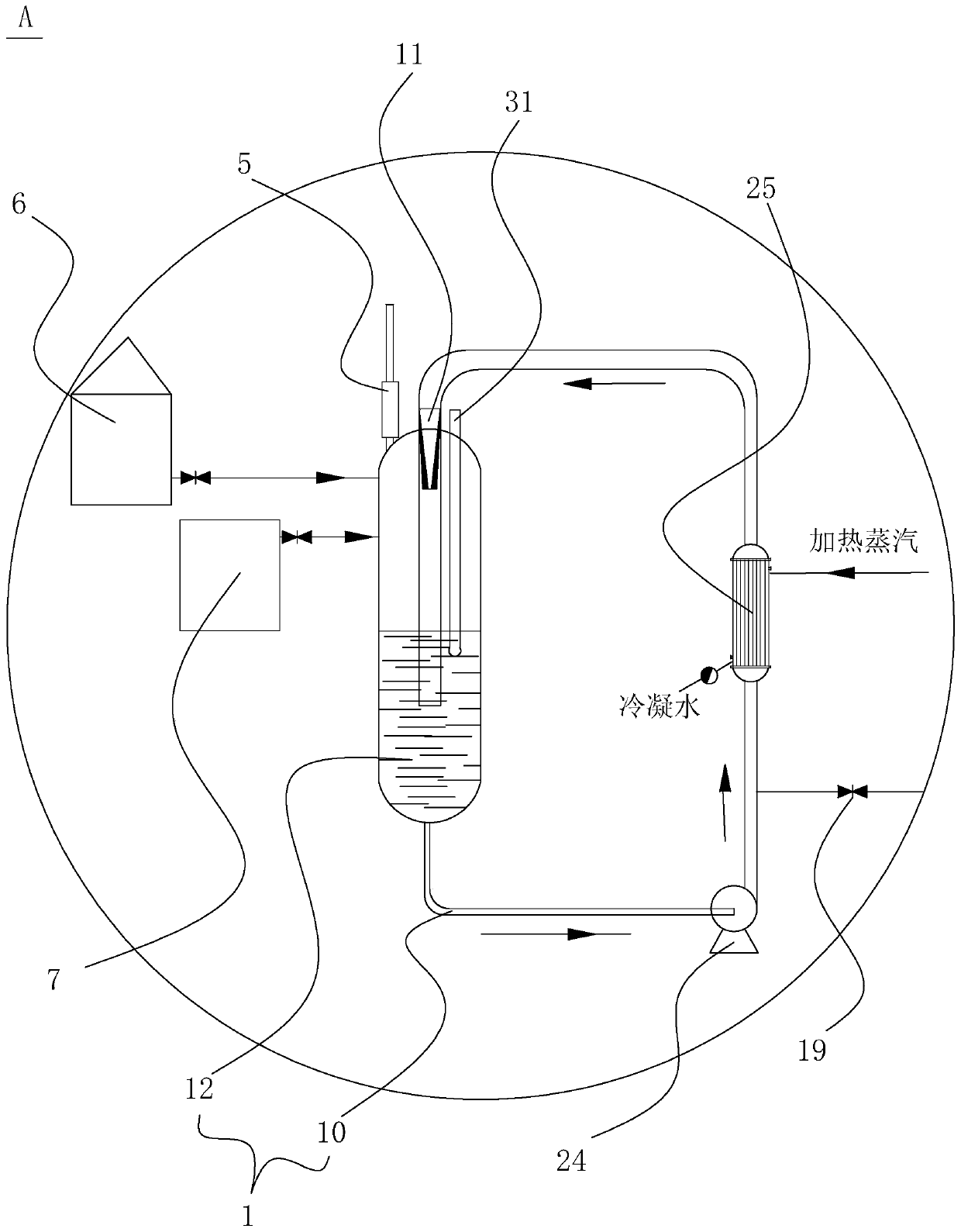
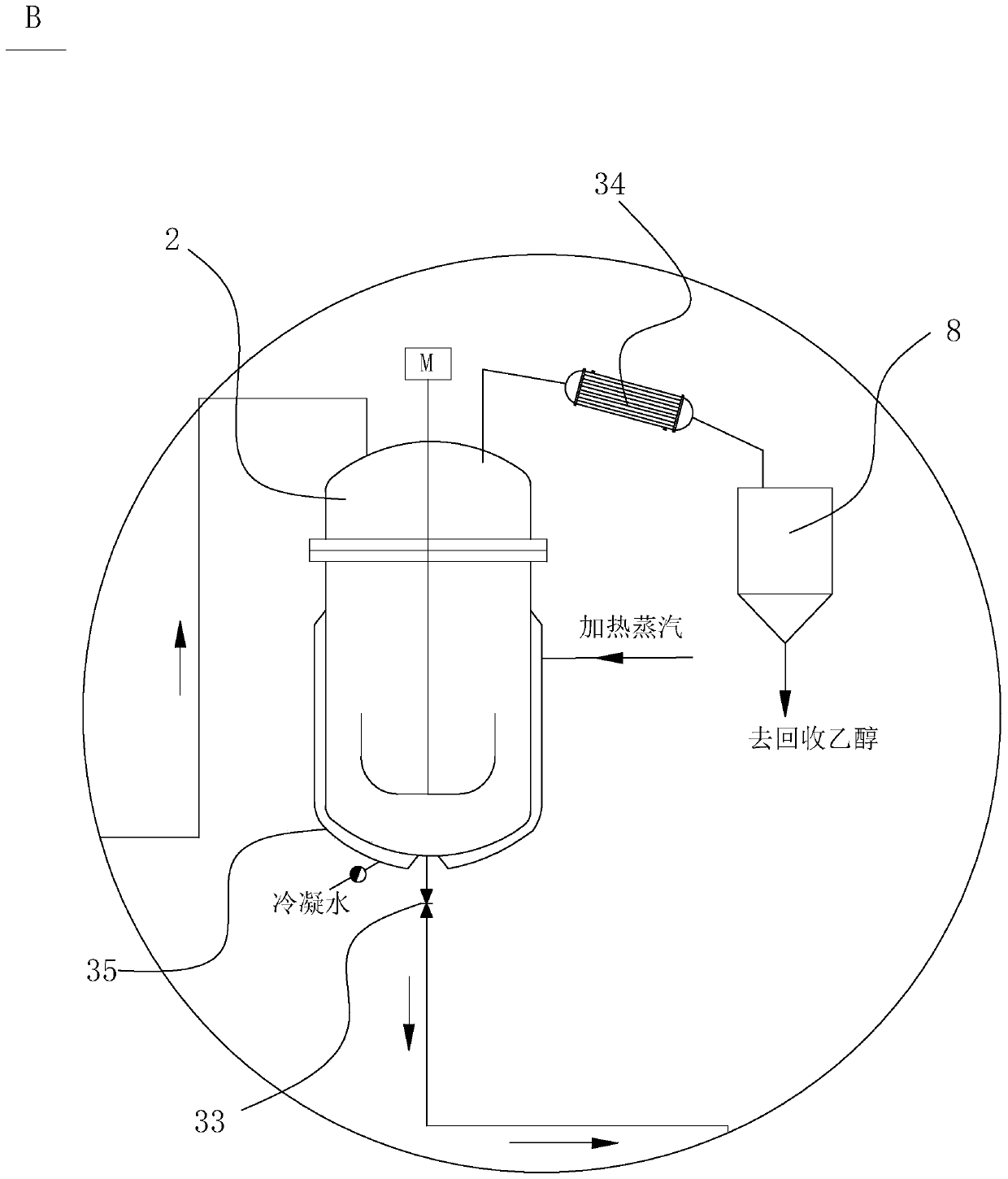
[0052] Put 1380Kg of ethanol into the esterification reactor 12, 100Kg of p-nitrobenzoic acid, 20Kg of concentrated sulfuric acid, open the circulation pump 24 of the esterification reactor 12, heat up to 75°C, and keep warm for 1 hour after the cycle residence time, then go to the est...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com