Alkenylation product of allylic alcohol carbamate compound and synthesis method thereof
A technology for allyl alcohol amine carboxylic acid and ester compounds, which is applied in the preparation of organic compounds, steroid compounds, organic chemical methods, etc., to achieve the effects of environmental friendliness, wide application range and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Palladium acetate (4.5 mg, 10 mol%, 0.02 mmol), N-acetylglycine (4.7 mg, 20 mol%, 0.04 mmol), silver carbonate (165.5 mg, 3.0 eq, 0.6 mmol) was charged to an oven-dried screw cap vial and trifluoroethanol (1.0 mL). Then, the corresponding carbamate (0.2 mmol, 1.0 equiv) and the corresponding alkene (0.40 mmol, 2.0 equiv) were sequentially added to the solution. The vial was sealed under nitrogen and heated to 80°C and stirred for 16 hours. After cooling, the mixture was filtered and concentrated to give the crude product, which was directly applied to flash column chromatography (ethyl acetate / petroleum ether mixture) to give bright yellow liquid (33.3 mg, yield 56%).
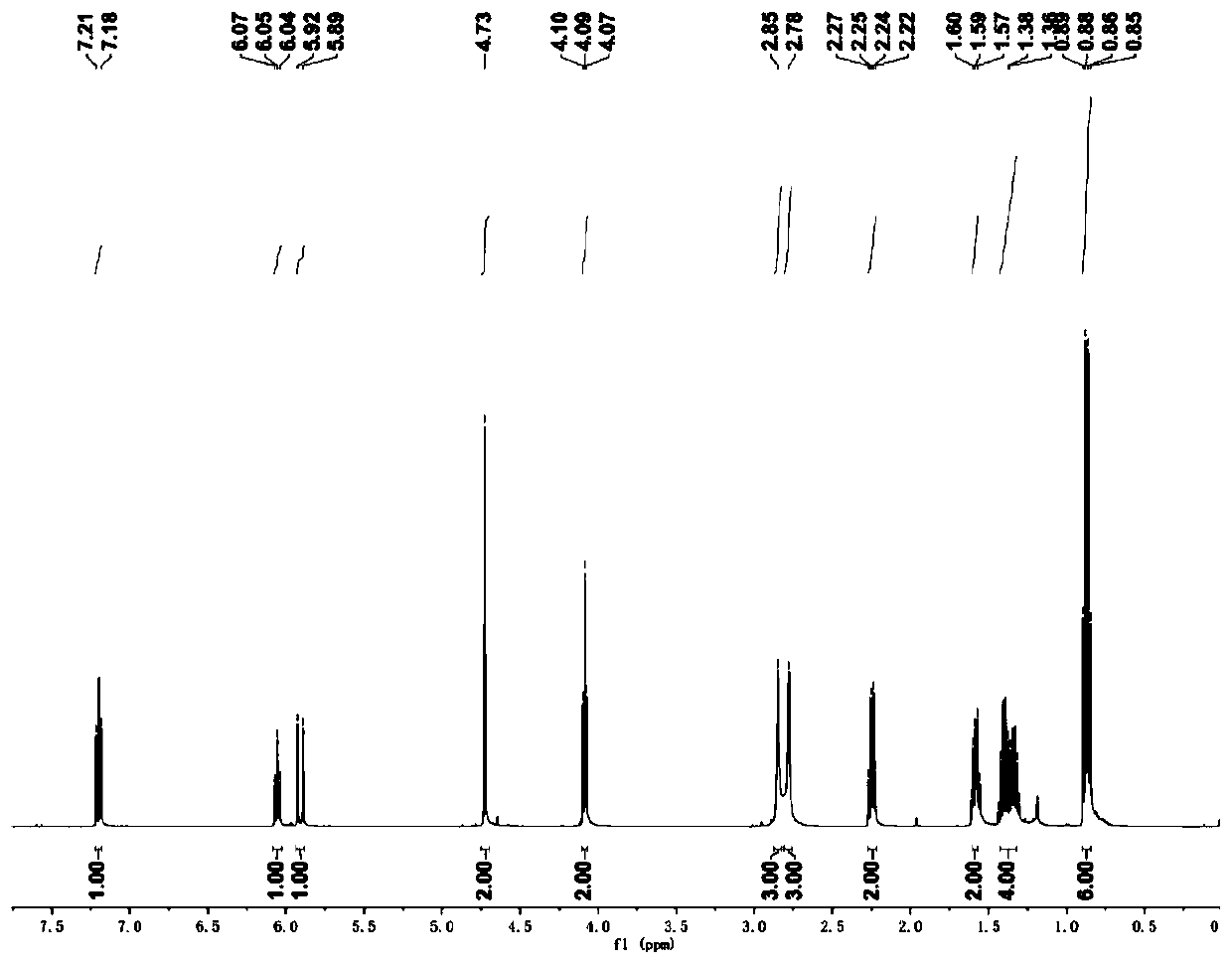
[0037] The NMR spectrum peaks are as follows: 1 H NMR (CDCl 3 ):δ=7.20(d,J=16.0Hz,1H),6.05(t,J=7.5Hz,1H),5.91(d,J=16.0Hz,1H),4.73(s,2H),4.09(t ,J=7.0Hz,2H),2.85(brs,3H),2.78(brs,3H),2.22-2.27(m,2H),1.56-1.61(m,2H),1.30-1.44(m,4H), 0.85-0.89(m,6H). 13 C NMR (CDCl 3): δ=166.52, 155.31, 1...
Embodiment 2
[0039]
[0040] Palladium acetate (4.5 mg, 10 mol%, 0.02 mmol), N-acetylglycine (4.7 mg, 20 mol%, 0.04 mmol), silver carbonate (165.5 mg, 3.0 eq, 0.6 mmol) was charged to an oven-dried screw cap vial and trifluoroethanol (1.0 mL). Then, the corresponding carbamate (0.2 mmol, 1.0 equiv) and the corresponding alkene (0.40 mmol, 2.0 equiv) were sequentially added to the solution. The vial was sealed under nitrogen and heated to 80°C and stirred for 16 hours. After cooling, the mixture was filtered and concentrated to give crude product, which was directly applied to flash column chromatography (ethyl acetate / petroleum ether mixture) to give bright yellow liquid (33.0 mg, yield 45%).
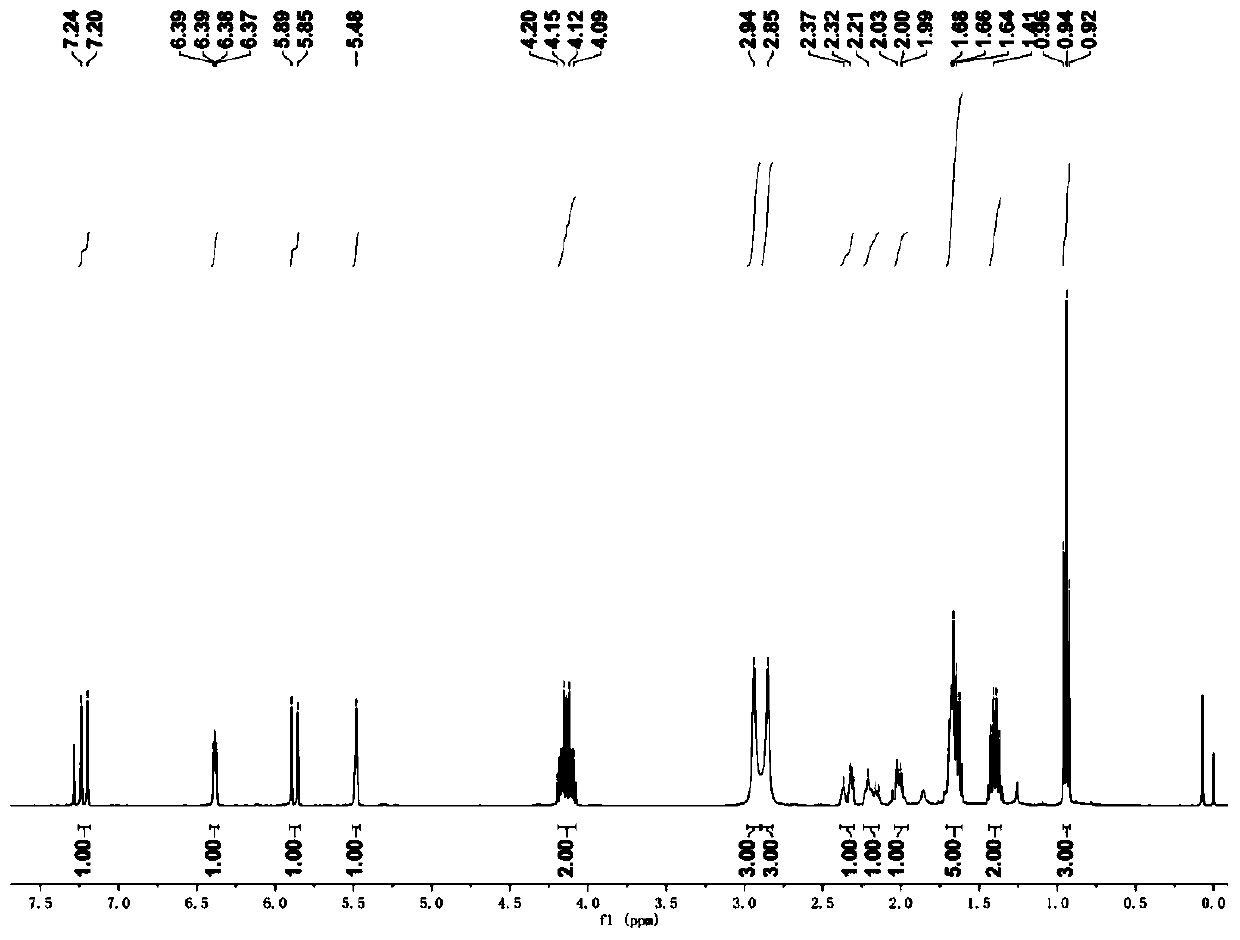
[0041] The NMR spectrum peaks are as follows: 1 H NMR (CDCl 3 ): δ=7.22(d, J=16.0Hz, 1H), 6.06(t, J=7.6Hz, 1H), 5.92(d, J=16.0Hz, 1H), 4.73(s, 2H), 3.68(s ,3H),2.85(brs,3H),2.78(brs,3H),2.22-2.28(m,2H),1.37-1.43(m,2H),0.86(t,J=7.6Hz,3H). 13 C NMR (CDCl 3 ): δ=167.85, 156.29, 146.96, 146.69,...
Embodiment 3
[0043]
[0044] Palladium acetate (4.5 mg, 10 mol%, 0.02 mmol), N-acetylglycine (4.7 mg, 20 mol%, 0.04 mmol), silver carbonate (165.5 mg, 3.0 eq, 0.6 mmol) was charged to an oven-dried screw cap vial and trifluoroethanol (1.0 mL). Then, the corresponding carbamate (0.2 mmol, 1.0 equiv) and the corresponding alkene (0.40 mmol, 2.0 equiv) were sequentially added to the solution. The vial was sealed under nitrogen and heated to 80°C and stirred for 16 hours. After cooling, the mixture was filtered and concentrated to give the crude product, which was directly applied to flash column chromatography (ethyl acetate / petroleum ether mixture) to give bright yellow liquid (24.8 mg, yield 46%).
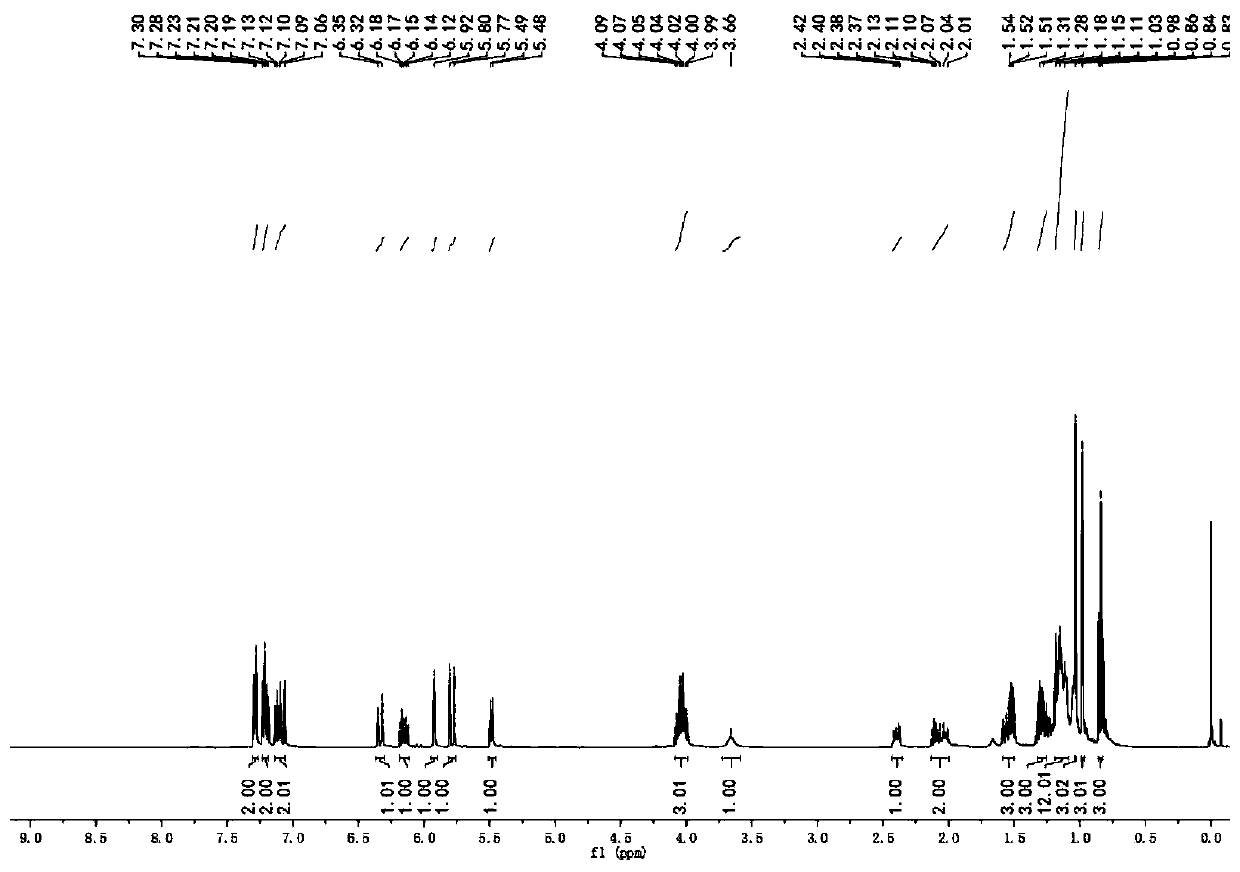
[0045] The NMR spectrum peaks are as follows: 1 H NMR (CDCl 3 ): δ=7.21(d, J=16.0Hz, 1H), 6.06(t, J=7.6Hz, 1H), 5.91(d, J=16.0Hz, 1H), 4.73(s, 2H), 4.14(q ,J=7.2Hz,2H),2.85(brs,3H),2.78(brs,3H),2.22-2.27(m,2H),1.37-1.43(m,2H),1.23(t,J=7.2Hz, 3H), 0.86(t, J=7.2Hz, 3H). 13 C NMR (CDCl 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com