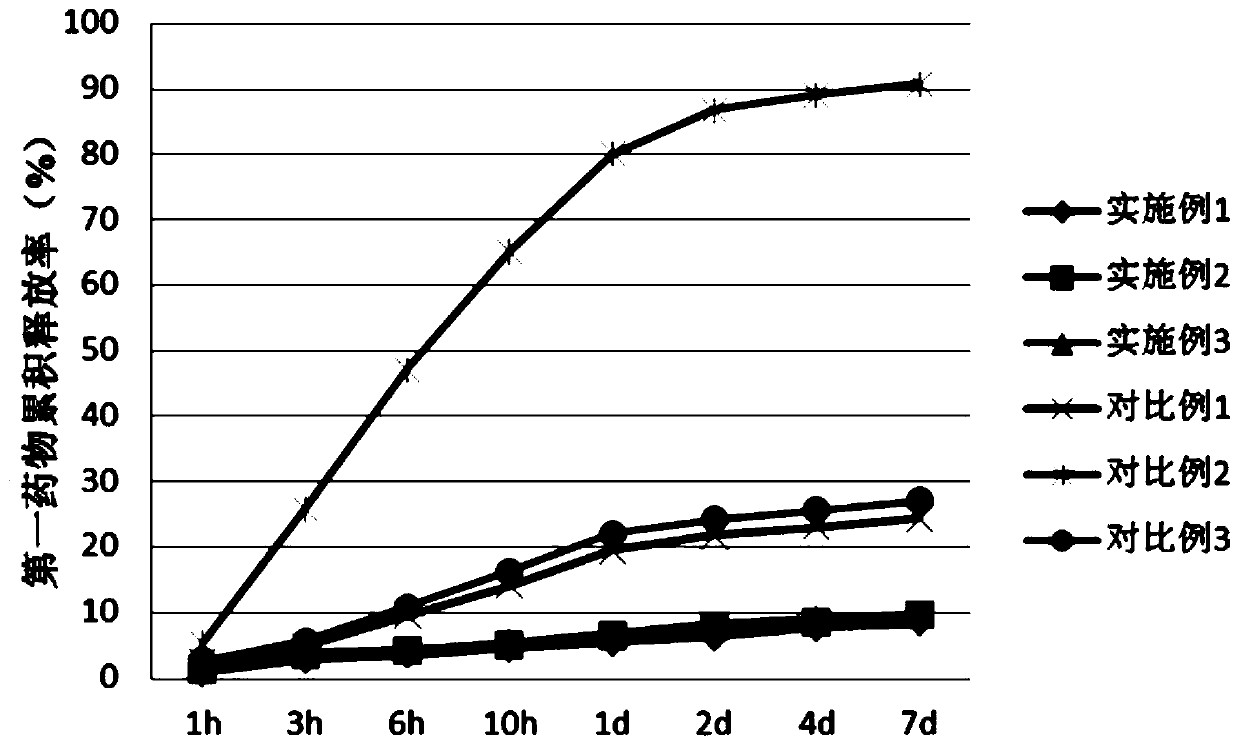
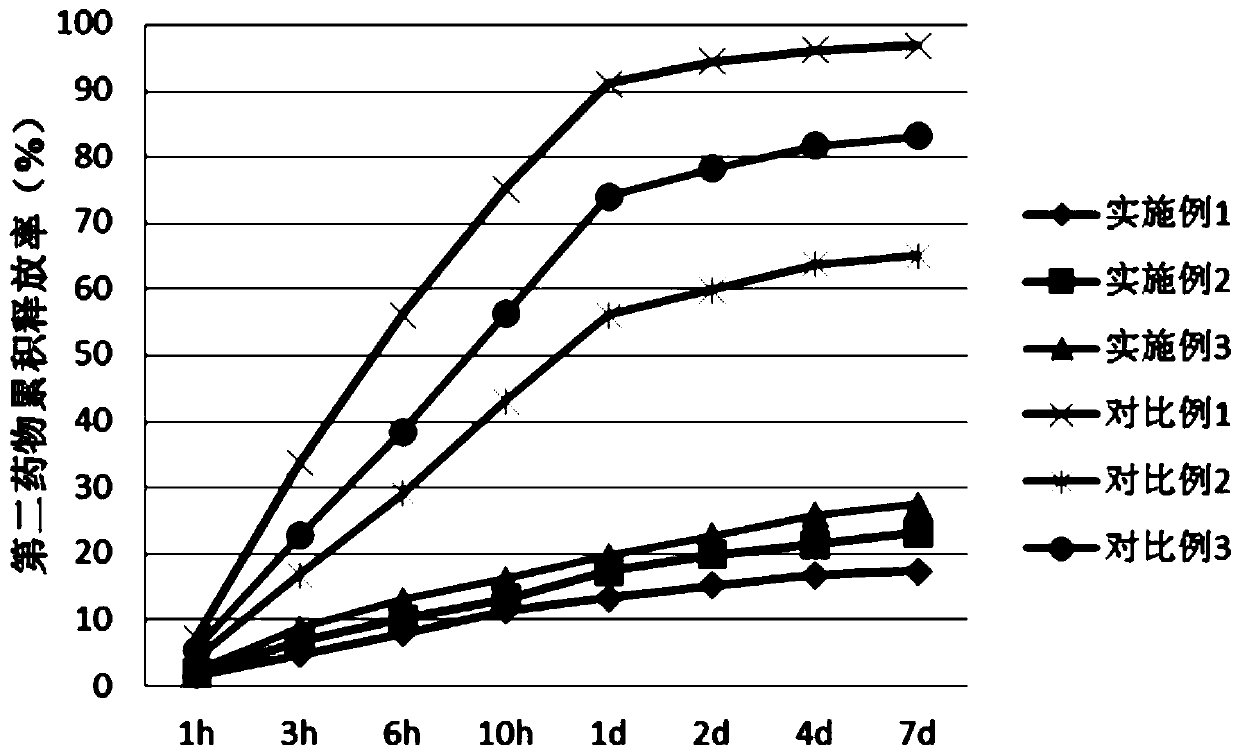
Polyleucine-polyaspartic acid block copolymer stereo-composite medicine carrying micelle and preparation method thereof
A technology of block copolymer and polyaspartic acid, which is applied in the field of biomedical materials, can solve the problems of short drug half-life and low encapsulation rate, and achieve the effect of not easy to escape, stable micelles, and enhanced therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1: Mix 20g of N-carboxy-L-leucine-anhydride in the ring with 1g of Co(PMe3)4 to form a reaction system. After cooling with liquid nitrogen, vacuumize, polymerize at 60°C for 24 hours in a nitrogen atmosphere, and then add 80gN -carboxyl-L-aspartic acid-cyclic acid anhydride to continue the polymerization reaction for 24 hours, the reaction product is filtered after precipitation treatment, and poly-L-amino acid block copolymer is obtained after drying;
[0029] Step 2: Mix 20g of N-carboxy-D-leucine-anhydride in the ring with 1g of Co(PMe3)4 to form a reaction system. After cooling with liquid nitrogen, vacuumize, polymerize at 60°C for 24 hours in a nitrogen atmosphere, and then add 80gN -carboxyl-L-aspartic acid-anhydride in the ring continued to polymerize for 24 hours, and the reaction product was filtered after precipitation treatment, and poly-D-amino acid block copolymer was obtained after drying;
[0030] Step 3: Cool 1g of poly-L-amino acid block copolymer...
Embodiment 2
[0034] Step 1: Mix 10g of N-carboxy-L-leucine-anhydride in the ring with 1g of Co(PMe3)4 to form a reaction system. After cooling with liquid nitrogen, vacuumize, polymerize at 20°C for 1 hour in a nitrogen atmosphere, and then add 190gN -carboxyl-L-aspartic acid-cyclic acid anhydride continued to polymerize for 48 hours, the reaction product was filtered after precipitation treatment, and poly-L-amino acid block copolymer was obtained after drying;
[0035] Step 2: Mix 10g of N-carboxy-D-leucine-anhydride in the ring with 1g of Co(PMe3)4 to form a reaction system. After cooling with liquid nitrogen, vacuumize, polymerize at 20°C for 1 hour in a nitrogen atmosphere, and then add 190gN -carboxyl-L-aspartic acid-cyclic acid anhydride continued to polymerize for 48 hours, and the reaction product was filtered after precipitation treatment, and poly-D-amino acid block copolymer was obtained after drying;
[0036]Step 3: Cool 1g of poly-L-amino acid block copolymer and 1g of poly-D...
Embodiment 3
[0039] Step 1: Mix 15g of N-carboxy-L-leucine-anhydride in the ring with 1g of Co(PMe3)4 to form a reaction system. After cooling with liquid nitrogen, vacuumize, polymerize at 100°C for 48 hours in a nitrogen atmosphere, and then add 30gN -carboxyl-L-aspartic acid-cyclic acid anhydride to continue the polymerization reaction for 1 hour, the reaction product is filtered after precipitation treatment, and poly-L-amino acid block copolymer is obtained after drying;
[0040] Step 2: Mix 15g of N-carboxy-D-leucine-anhydride in the ring with 1g of Co(PMe3)4 to form a reaction system. After cooling with liquid nitrogen, vacuumize, polymerize at 100°C for 48 hours in a nitrogen atmosphere, and then add 30gN -carboxyl-L-aspartic acid-cyclic acid anhydride to continue the polymerization reaction for 1h, the reaction product is filtered after precipitation treatment, and the poly-D-amino acid block copolymer is obtained after drying;
[0041] Step 3: Cool 1g of poly-L-amino acid block c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com