(S)-BIONL derivative CSP filler and preparation method and application thereof
A technology of -BIONL and S-6-ABINOL, which is applied in the field of -BIONL derivative CSP filler and its preparation, can solve problems such as obstacles and inability to separate chiral compounds, and achieve good stability, stable chiral recognition ability, Meet the effect of pharmaceutical analysis and production quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention also provides a preparation method of the (S)-BIONL derivative CSP filler described in the above technical scheme, comprising the following steps:
[0042] Carry out hydroxyl protection to S-6-A BINOL, obtain the compound with the structure shown in formula II;
[0043]
[0044] Condensing a compound having a structure shown in formula II with a silylating agent to obtain a condensation product;
[0045] The condensation product is bonded to an acidified silica gel carrier, and then subjected to dehydroxylation protection to obtain the (S)-BIONL derivative CSP filler.
[0046] The present invention carries out hydroxyl protection on S-6-A BINOL to obtain a compound having a structure shown in formula II (abbreviated as (S)-1);
[0047]
[0048] The present invention preferably carries out structural modification to the BINOL of S configuration, obtains S-6-acrylic acid BINOL (S-6-A BINOL) at the 6-position substitution acrylic acid of naphth...
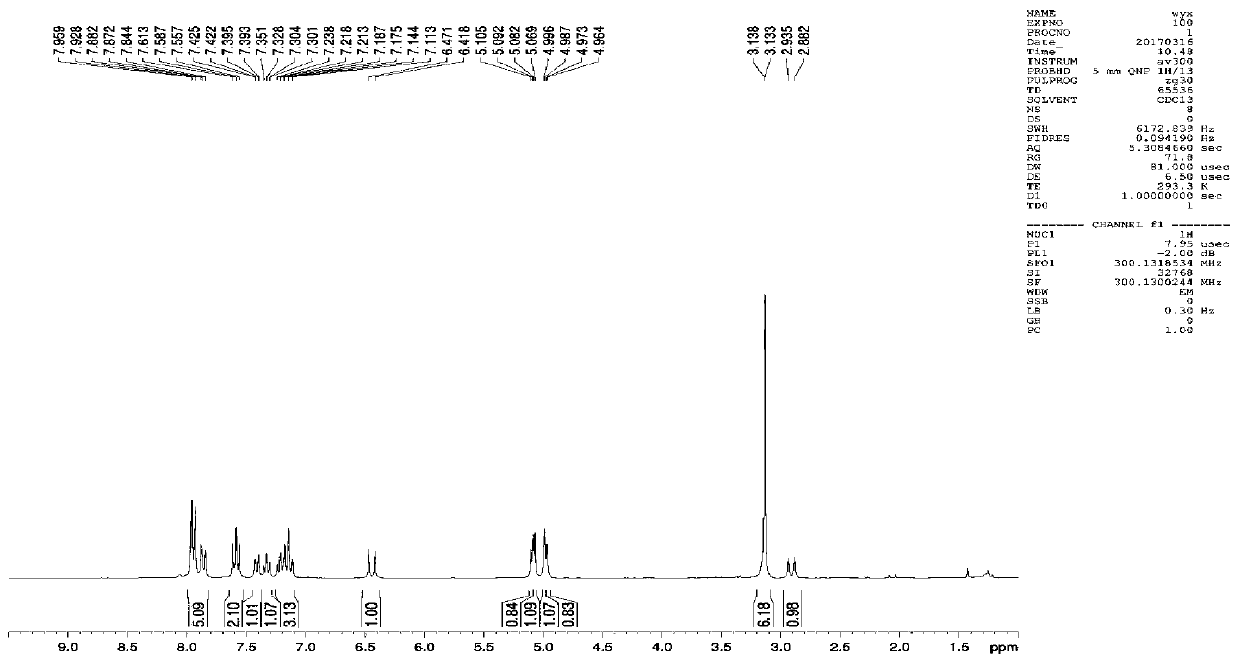
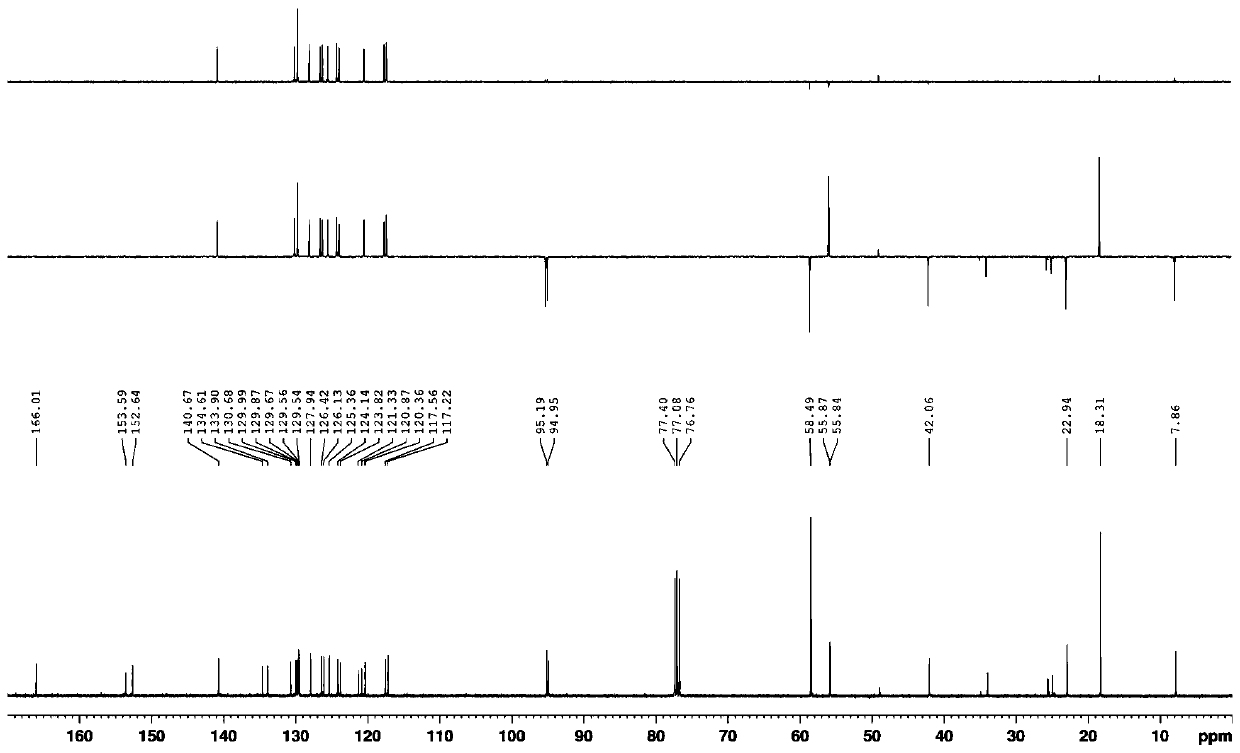
Embodiment 1
[0079] This example is to modify the structure of S-configuration BINOL. Substitute acrylic acid at the 6-position of the naphthalene ring to obtain S-6-acrylic acid BINOL (S-6-A BINOL). For 1 and 1 of S-6-A BINOL Carry out hydroxyl protection at the ' position to obtain compound (S)-1, (S)-1 is bonded to the acidified silica gel carrier after silanization, and then removes hydroxyl protection to obtain S-6-acrylic acid BINOL chiral fixed packing (S -6-A BINOL CSP).
[0080] In a 50mL single-necked bottle, dissolve the derivative (S)-1 (1.125mmol, 500mg) in anhydrous dichloromethane (15mL), then add triethylamine (3.375mmol, 0.47mL), and stir in an ice bath After 5 minutes 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate (1.2375 mmol, 470.25 mg) was added. After removing the ice and stirring at room temperature for 30 minutes, 3-aminopropyltriethoxysilane (1.125 mmol, 0.26 mL) was added and reacted at room temperature for 3 hours, and the reaction wa...
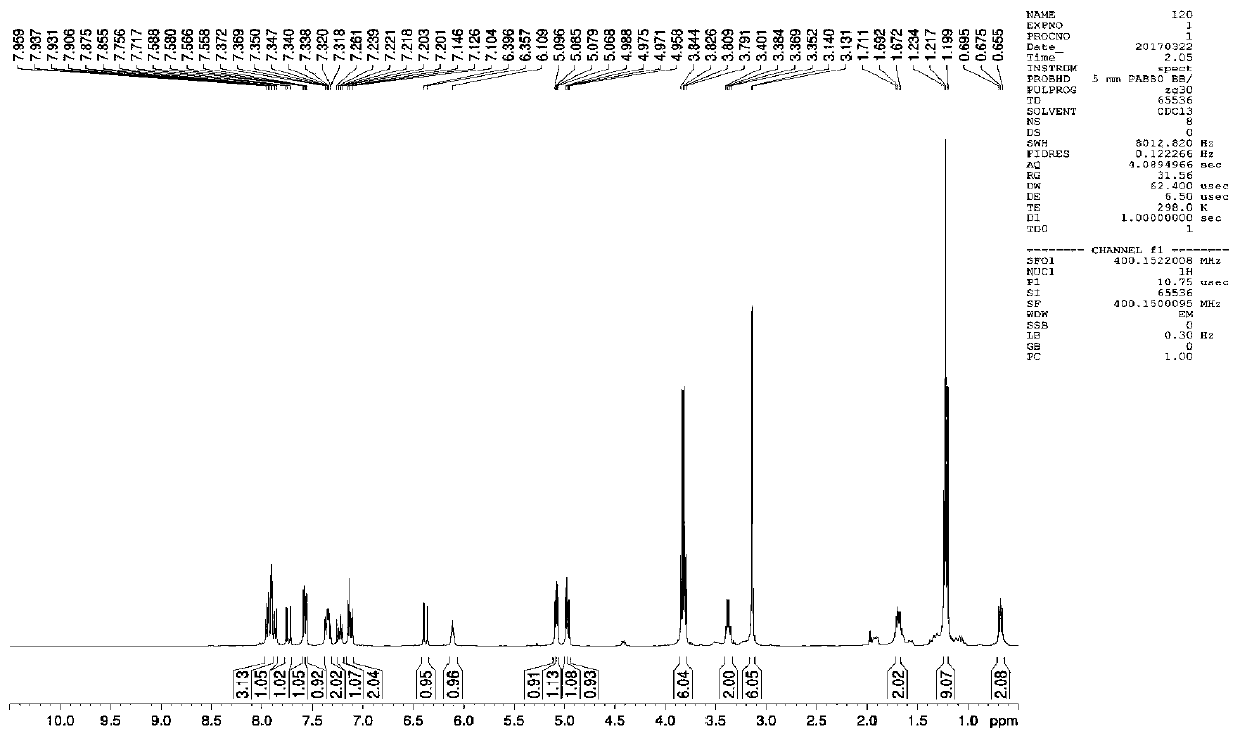
Embodiment 2
[0093] This example is to modify the structure of S-configuration BINOL. Substitute acrylic acid at the 6-position of the naphthalene ring to obtain S-6-acrylic acid BINOL (S-6-A BINOL). For 1 and 1 of S-6-A BINOL The hydroxyl group was protected at the ' position to obtain compound (S)-1, and the compound (S)-1 was bonded to an aminopropyl silica gel carrier, and finally dehydroxylated to obtain S-6-A BINOL CSP.
[0094] Take 10g of silica gel, 100mL of 4M hydrochloric acid, N 2 Reflux for 5 hours, cool to room temperature, wash the silica gel with ultrapure water until neutral, and dry it in vacuum at 140°C for 24 hours to obtain acidified silica gel, which is stored in a sealed and cool place for later use. Acidify silica gel (3.5g) and add it into a 250mL three-necked flask filled with 100mL of dry toluene. The toluene takes out the residual moisture and cools to room temperature. According to the specific surface area of silica gel, add 2 times the amount of 3-aminopro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com