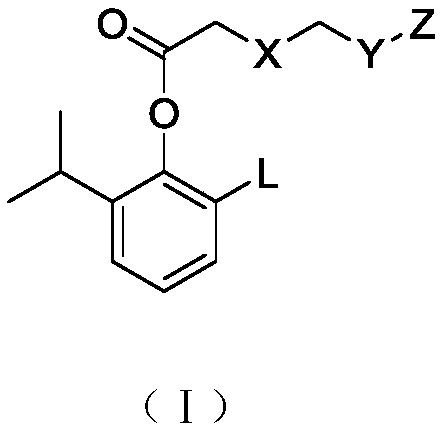
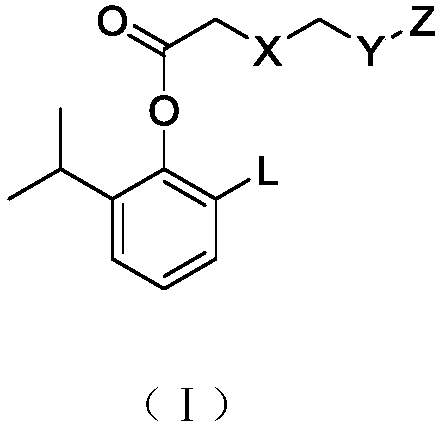
Alkyl-substituted phenol carboxylate compound, preparation method and application
A technology of phenol esters and compounds, applied in the field of alkyl substituted phenol carboxylate compounds, preparation and application, capable of solving problems such as drug accumulation, poor stability, and limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017]
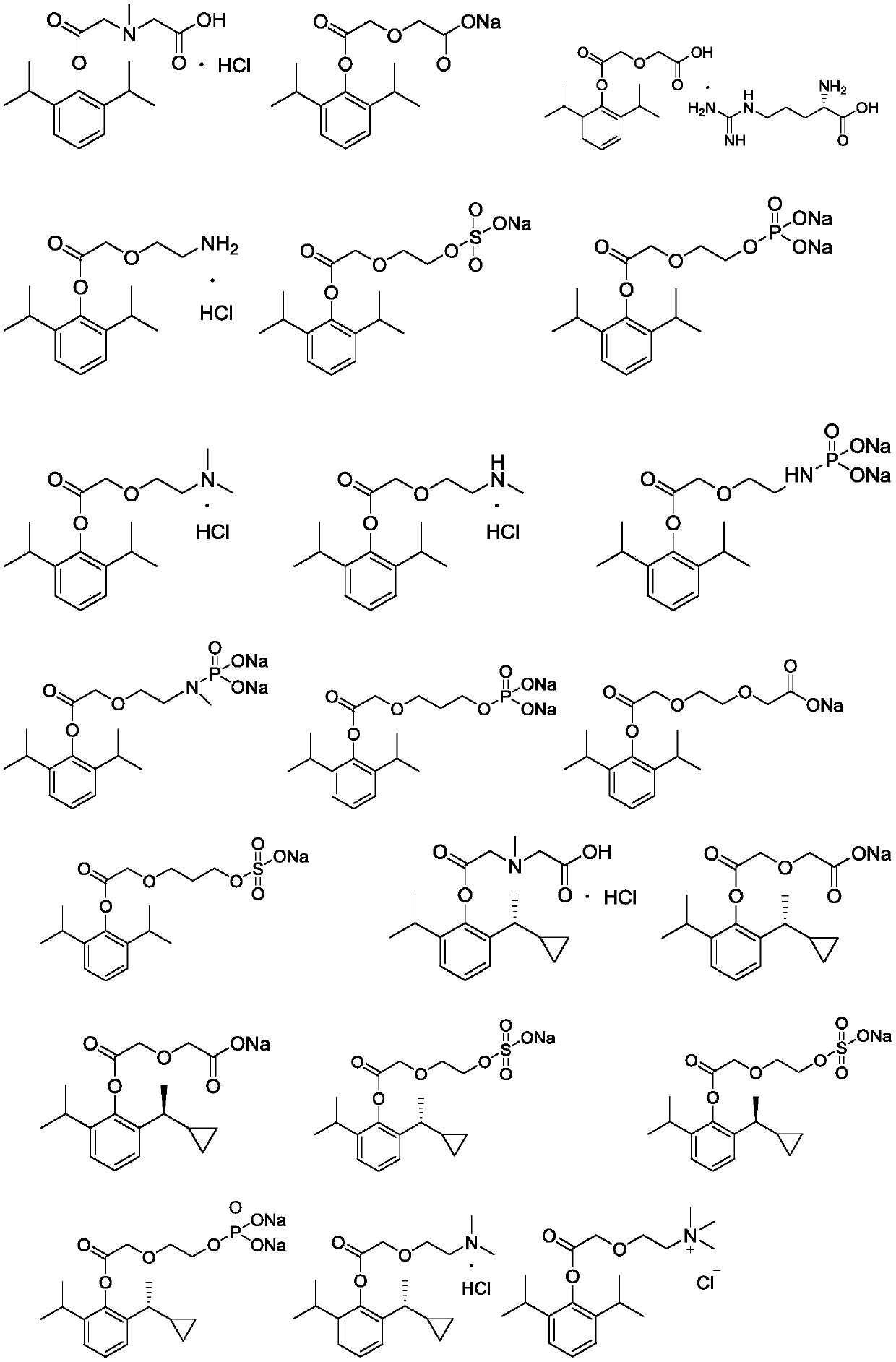
[0018] Dissolve 1.47g of N-methyliminodiacetic acid (CAS: 4408-64-4) and 1.78g of propofol in 30mL of N,N-dimethylformamide, add 2.06g of N,N-dicyclohexyl Carbodiimide (DCC), stirred at room temperature for 2 hours, filtered, evaporated to dryness under reduced pressure of DMF, added 50mL of 2N hydrochloric acid to the residue, filtered, evaporated to dryness of the filtrate under reduced pressure, and obtained 1.92 g of white solid by column chromatography, namely Compound 1, yield: 55.2%.
[0019] 1 HNMR (D 2 O,400MHz) δ: 1.17~1.19(12H,d,J=8Hz), 2.83(3H,s), 2.92(2H,p,J=8Hz), 4.12(2H,s), 4.43(2H,s) ,7.32~7.37(3H,m).
Embodiment 2
[0021]
[0022] Dissolve 1.34g of diglycolic acid (CAS: 110-99-6) and 1.78g of propofol in 30mL of N,N-dimethylformamide, add 2.06g of N,N-dicyclohexylcarbodiimide (DCC), stirred at room temperature for 2 hours, filtered, evaporated to dry DMF under reduced pressure, added 50mL 2N hydrochloric acid to the residue, precipitated, filtered, suspended the precipitate with 10mL water, adjusted the pH value to 8.5 with 0.5N aqueous sodium hydroxide solution, added 20mL of acetone precipitated a white solid and filtered to obtain 1.08g of compound 2 with a yield of 36.6%.
[0023] 1 HNMR (D 2 O,400MHz) δ: 1.17~1.19(12H,d,J=8Hz), 2.91(2H,p,J=8Hz), 4.11(2H,s), 4.68(2H,s), 7.31~7.39(3H, m).
Embodiment 3
[0025]
[0026] Dissolve 2.19g of 5-tert-butoxycarbonylamino-3-oxavaleric acid (CAS: 142929-49-5) and 1.78g of propofol in 30mL of N,N-dimethylformamide, add 2.06g of N , N-dicyclohexylcarbodiimide (DCC), stirred at room temperature for 2 hours, filtered, evaporated to dryness in DMF under reduced pressure, added 20mL 20% methanolic hydrochloric acid solution to the residue and stirred at room temperature for 2 hours, filtered, evaporated to dryness, and the residue was Column chromatography yielded 1.21 g of a white solid, namely compound 3, with a yield of 38.4%.
[0027] 1 HNMR (D 2 O,400MHz) δ:1.16~1.19(12H,d,J=8Hz),2.91(2H,p,J=8Hz),3.45~3.52(2H,m),4.12~4.18(2H,m),4.56( 2H, s), 7.31 ~ 7.39 (3H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com