A main chain type sulfonated polyquinoxaline prepared by post-sulfonation method and its proton exchange membrane
A technology of sulfonated polyquinoxaline and main chain type, applied in the field of proton exchange membrane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
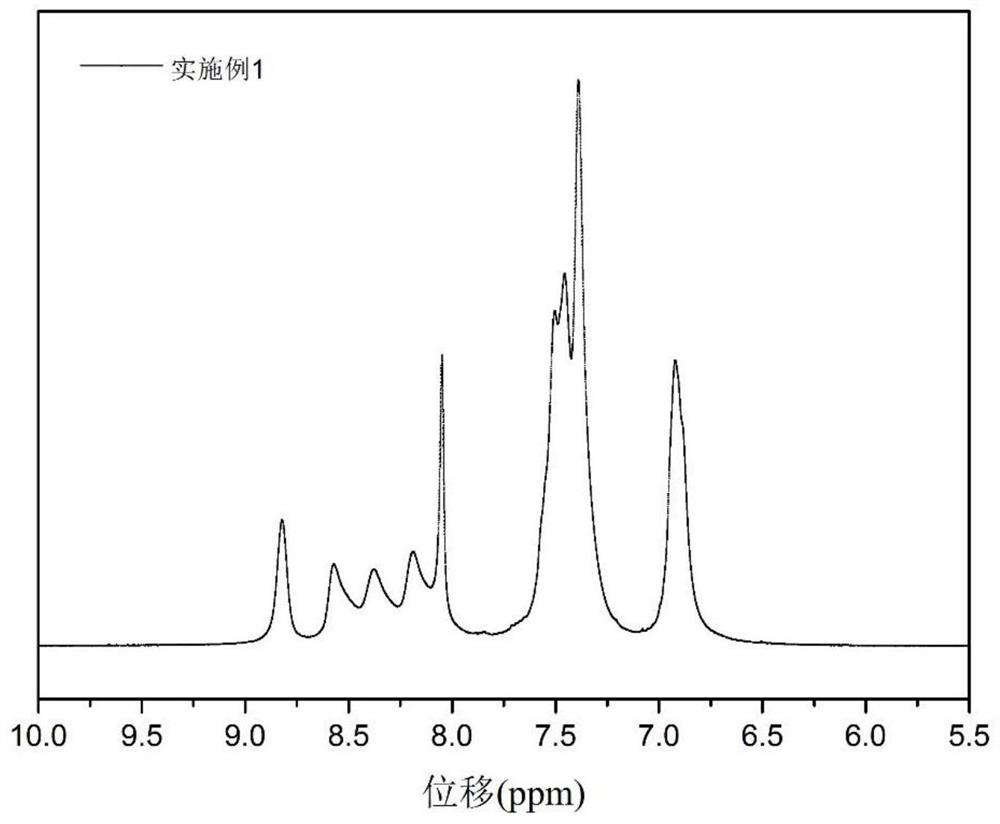
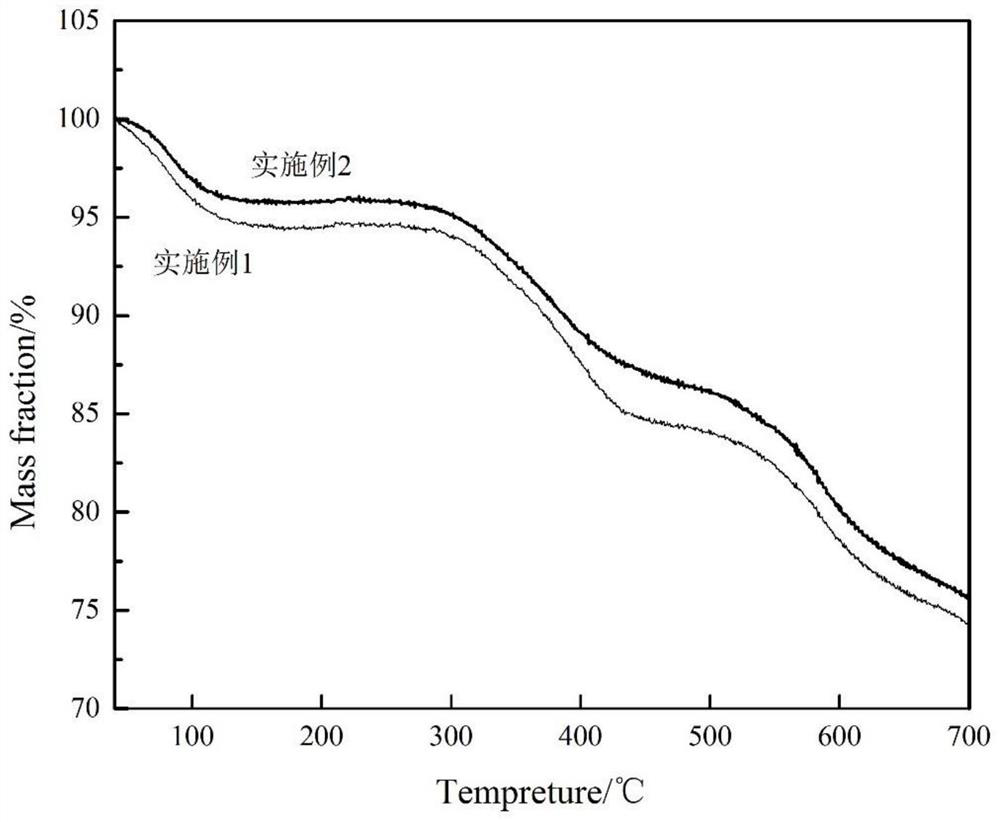
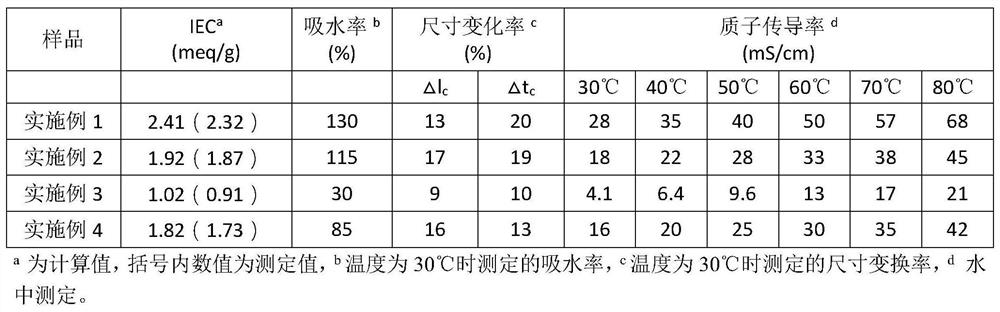
Embodiment 1
[0034] In this embodiment, the tetraamine (1) skeleton is used as an example to synthesize sulfonated polyquinoxaline from the monomer (4) of the polyquinoxaline structural unit (a) before sulfonation, and the structure is as follows:
[0035]
[0036] The specific preparation method is:
[0037] (1) Polymerization
[0038] Weigh 1.2052g of 4,4'-bis(4-(2-(4-phenyl)acyl)phenoxy)biphenyl and 0.4285g of 3,3',4,4'-tetraaminobiphenyl respectively, and mix Then pour it into a flask, add m-cresol to dissolve, react at room temperature for about 24 hours under a nitrogen atmosphere, pour the solution into ethanol to precipitate the polymer, wash repeatedly until it is clean, filter, collect the solid, and dry it in vacuum to obtain polymer fibers.
[0039] (2) Sulfonation
[0040] Pour the polymer fibers into a flask, add fuming sulfuric acid at a ratio of 10-15wt% solid content, react under a nitrogen atmosphere, pour the liquid into water, the sulfonated product is precipitated...
Embodiment 2
[0044] In this embodiment, the monomer (4) of the polyquinoxaline structural unit (a) and the monomer (3) of the structural unit (b) before sulfonation are used as raw materials to synthesize sulfonated polyquinoxaline with the tetraamine (1) skeleton Taking morphine as an example, the structure is as follows (wherein x:y=3:1):
[0045]
[0046] The specific preparation method is:
[0047] (1) Polymerization
[0048] Weigh 0.9039g of 4,4'-bis(4-(2-(4-phenyl)acyl)phenoxy)biphenyl, 4,4'-bis(2-(4-phenyl)acyl) Diphenyl ether 0.2172g and 3,3',4,4'-tetraaminobiphenyl 0.4285g, add m-cresol to dissolve, react at room temperature under nitrogen atmosphere for about 24h, after the reaction, pour the solution into ethanol to make the polymer The precipitates are washed repeatedly until they are clean, filtered, and dried to obtain polymer fibers.
[0049] (2) Sulfonation
[0050] React the obtained polymer with concentrated sulfuric acid, pour the solution into water after the react...
Embodiment 3
[0054] In this embodiment, the tetraamine (3) skeleton is used as the raw material to synthesize the sulfonated polyquinoxaline monomer (1) of the structural unit (a) and the monomer (1) of the structural unit (b) before sulfonation. Taking quinoxaline as an example, the structure is as follows (wherein x:y=3:1):
[0055]
[0056] The specific preparation method is:
[0057] (1) Polymerization
[0058] Weigh 0.7898g of 1,4-bis(4-(2-(4-phenyl)acyl)phenoxy)benzene, 0.2092g of biphenylil and 3,3',4,4'-tetraaminobis 0.4605g of phenylene ether was dissolved by adding m-cresol, and reacted at room temperature under nitrogen atmosphere for about 24 hours. After the reaction, the solution was poured into ethanol to precipitate the polymer, washed repeatedly until it was cleaned, filtered, and dried to obtain polymer fibers.
[0059] (2) Sulfonation
[0060] Pour the polymer fibers into a flask, add fuming sulfuric acid at a ratio of 10-15wt% solid content, react under a nitrogen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com