Amphiphilic temperature-sensitive block polymer base on phenylboronic acid and preparation method and application thereof
A temperature-sensitive block and amphiphilic technology, applied in the field of fractional purification of natural products, can solve the problems of time-consuming procedures, limited large-scale application, complex operation, etc., and achieve good compatibility and fast association/desorption kinetics Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Thermosensitive Polymer PEG 113 -b-PVBA 49 -b-PNIPAM 105 preparation of
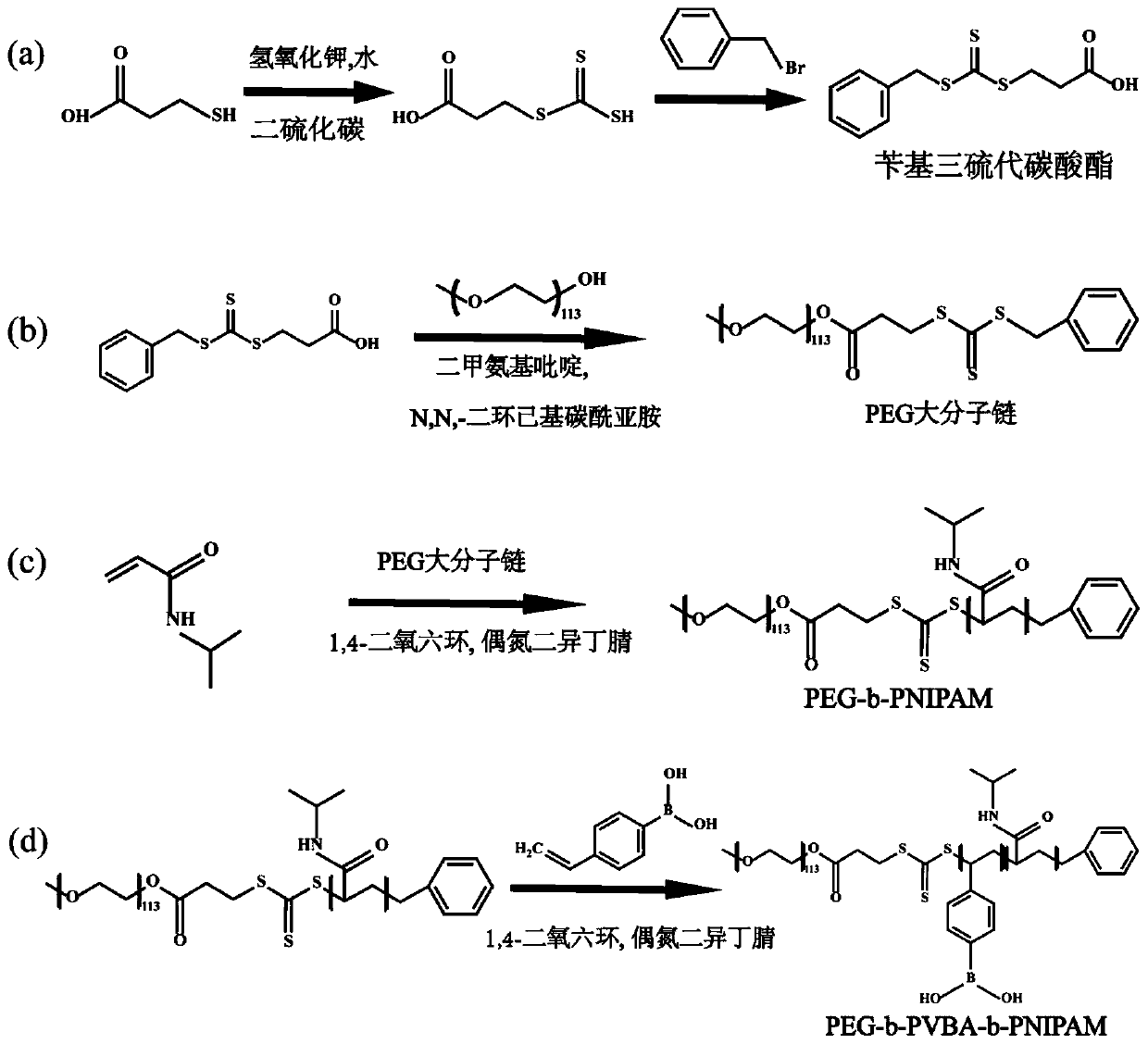
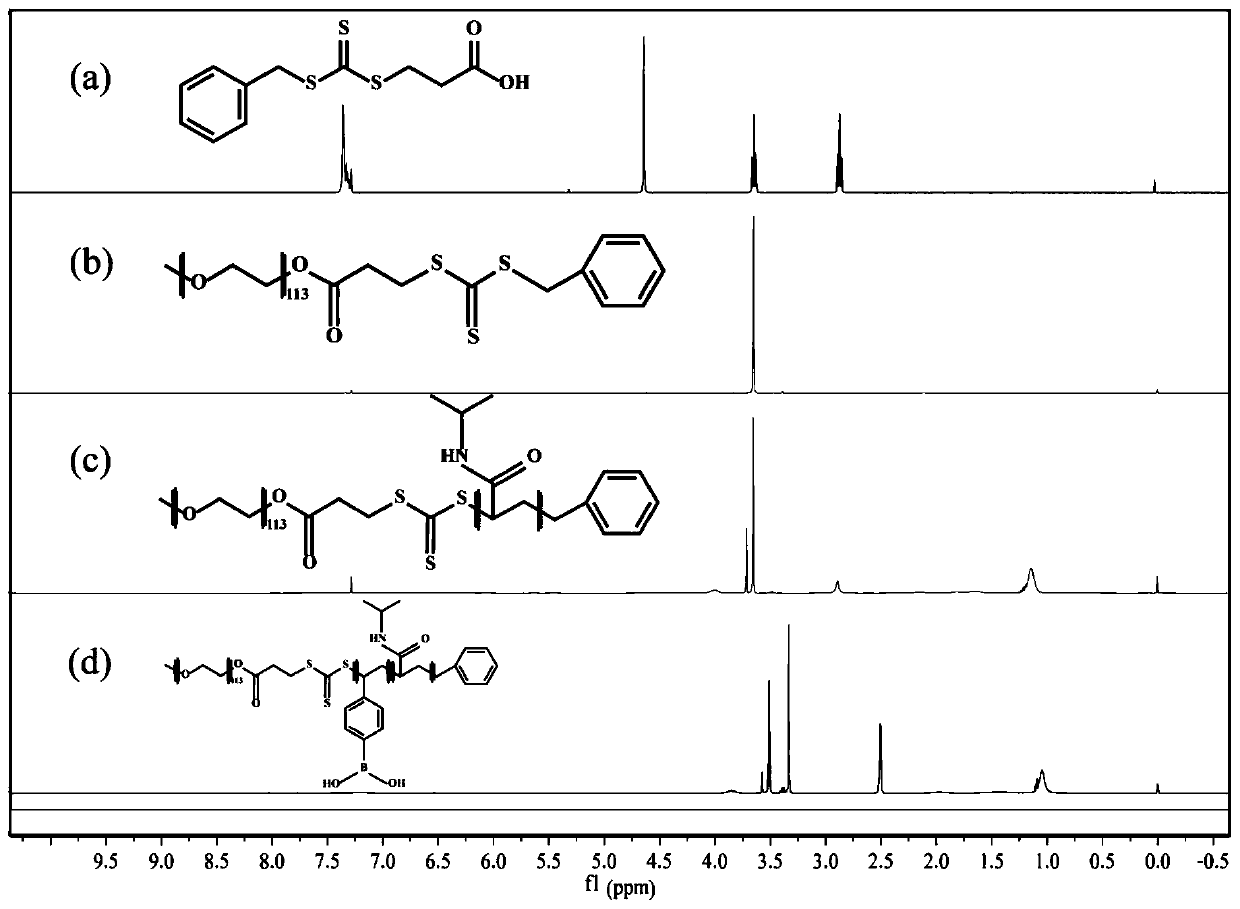
[0067] Synthesis of Triblock Copolymer PEG Using Reversible Addition-Fragmentation Chain Transfer Polymerization Mechanism 113 -b-PVBA 49 -b-PNIPAM 105 . First synthesize the PEG macromolecular chain, and then add the PNIPAM block and the BA block to the polymer in sequence. The detailed synthesis flow chart is as follows: figure 1 , in the figure a is the synthesis process of BTPA, b is the synthesis process of PEG macromolecular chain, and c is the synthesis process of PEG 113 -b-PNIPAM 105 The preparation process, d is PEG 113 -b-PVBA 49 -b-PNIPAM 105 preparation process. Specific steps are as follows:
[0068] (1) Preparation of benzyl trithiocarbonate (BTPA):
[0069]
[0070] Add 5.00 mL of 3-mercaptopropionic acid (MPA, 28.65 mmol) into 65.00 mL of KOH (1.84 mol / L) aqueous solution, and then add 3.65 mL of CS 2Slowly drop into the above mixed solution; then react...
Embodiment 2
[0090] Example 2: Thermosensitive Polymer PEG 113 -b-PVBA 49 -b-PNIPAM 105 preparation of
[0091] The preparation steps of benzyl trithiocarbonate (BTPA) and PEG macromolecular chains are the same as step (1) and step (2) in Example 1. Then include the following steps:
[0092] PEG 113 -b-PNIPAM 105 Preparation: Synthesize amphiphilic block copolymers by modifying PEG macromolecular chain transfer agent, including:
[0093] Put N-isopropylacrylamide, PEG macromolecular chains, azobisisobutyronitrile and 1,4-dioxane into a reaction vessel equipped with magnetic stirring; remove the air in the reaction vessel, and the solution is Protected oil bath reaction; after the reaction is completed, the solution is frozen in the refrigerator, and excess ether is used to remove impurities to obtain light yellow powder, which is PEG 113 -b-PNIPAM 105 , where b represents the combination of PEG macromolecular chains and PNIPAM in the form of block polymerization.
[0094] Wherein,...
Embodiment 3
[0101] Example 3: Thermosensitive Polymer PEG 113 -b-PVBA 49 -b-PNIPAM 105 preparation of
[0102] The preparation steps of benzyl trithiocarbonate (BTPA) and PEG macromolecular chains are the same as step (1) and step (2) in Example 1. Then include the following steps:
[0103] PEG 113 -b-PNIPAM 105 Preparation of:
[0104] Synthesis of amphiphilic block copolymers by modifying PEG macromolecular chain transfer agents, including:
[0105] Put N-isopropylacrylamide, PEG macromolecular chains, azobisisobutyronitrile and 1,4-dioxane into a reaction vessel equipped with magnetic stirring; remove the air in the reaction vessel, and the solution is Protected oil bath reaction; after the reaction is completed, the solution is frozen in the refrigerator, and excess ether is used to remove impurities to obtain light yellow powder, which is PEG 113 -b-PNIPAM 105 , where b represents the combination of PEG macromolecular chains and PNIPAM in the form of block polymerization.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com