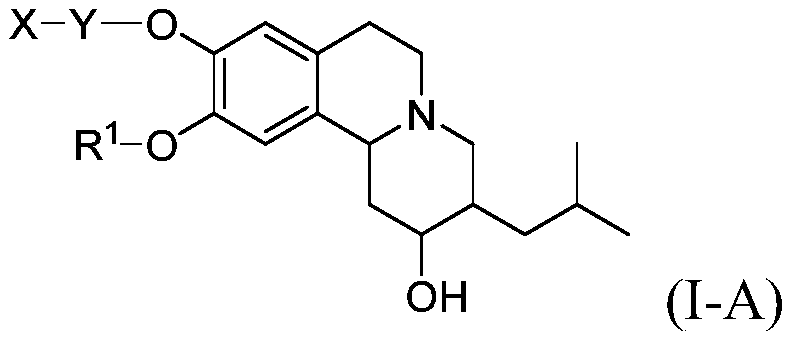
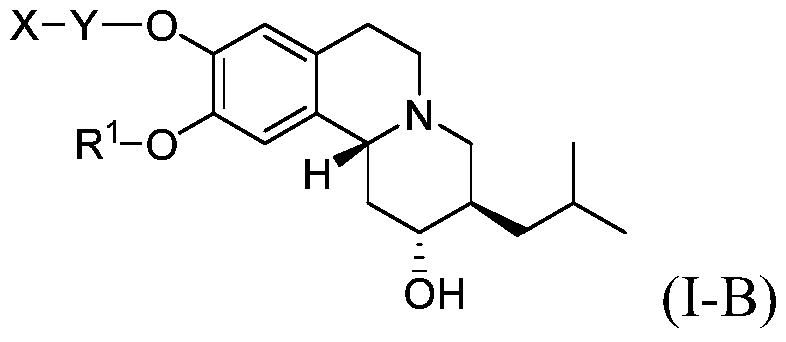
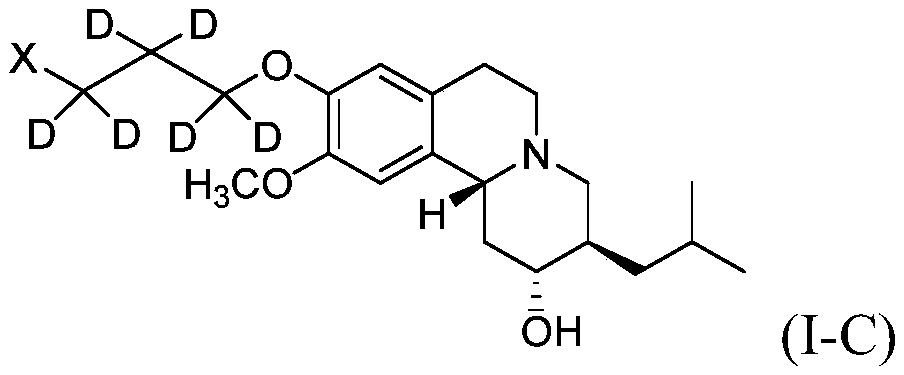
Novel deuterium substituted positron emission tomography (PET) imaging agents and their pharmacological application
A technology of pharmacy and deuterium atoms, which is applied in the direction of radioactive preparations in vivo, echo chromatography, drug combination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0234] Synthesis of Compound Ia
[0235] Option 6
[0236]
[0237] Synthesis of compound Ia-2: (2R,3R,11R)-3-isobutyl-10-methoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1- a] Isoquinoline-2,9-diol
[0238] A mixture of 9-benzyl protected DTBZ (Ia-1, 380 mg, 0.96 mmol) and 10% dry Pd / C (15 mg) in THF (10 mL) and EtOH (5 mL) was dissolved in H 2 Stirring was continued for 6 hours. The reaction mixture was filtered and washed with EtOH (10 mL) and THF (10 mL). The solvent was removed in vacuo to afford Ia-2 (255 mg, 87%) as a yellow solid. 1 HNMR (400MHz, CDCl 3 )δ6.68(s,1H),6.67(s,1H),3.87(s,3H),3.44-3.38(m,1H),3.16-2.97(m,4H),2.66-2.56(m,2H) ,2.49-2.42(m,1H),1.99(t,J=2.01Hz,1H),1.79-1.68(m,2H),1.57-1.45(m,3H),1.12-1.05(m,1H),0.97 -0.93(m,6H). C 18 h 27 NO 3 [M+H] + The HRMS calculated value is 306.2069 and the measured value is 306.2100.
[0239]
[0240] Synthesis of Compound Ia-4: [1,1,2,2,3,3-D 6 ]-propane-1,3-diylbis(4-methylbenzenesulfonate)
[0241] To a...
Embodiment 2
[0251] In Vitro Binding Assay for Ki Determination of AV-133 Relative to AV-133-D6 (Ia)
[0252] Tissue homogenates of striatum (excised from rat brains) were prepared in 50 mM HEPES (pH 7.5) and 0.3 M sucrose. Check compound in 10 -7 to 10 -12 Competing for binding in the M concentration range 18 F-AV-133 or 18 F-AV-133-D6( 18 F-Ia) (0.15-0.2 nM) capacity. Binding assays were performed in glass tubes (12 x 75mm) with a final volume of 0.25 mL. Non-specific binding was defined with 10 μM (±)-tetrabenazine (TBZ). After incubation at room temperature for 90 min, bound ligand was separated from free ligand by filtration through a glass fiber filter. Wash the filter three times with 4 mL of ice-cold PBS buffer (pH 7.4), and use a gamma counter (WIZARD 2 , Perkin-Elmer) counted the radioactivity on the filter. Data were analyzed to determine IC using the nonlinear least squares curve fitting program LIGAND 50 , and Ki was calculated by the Cheng-Prusoff equation using 0.1...
Embodiment 3
[0259] Comparison of FP-DTBZ biodistribution data in rats: 18 F-FP-(+)DTBZ vs. 18 F-FP-DTBZ-D6( 18 F-Ia)
[0260]
[0261] Three rats per group were used for each biodistribution study. Under isoflurane anesthesia, inject 0.2 mL of saline solution containing 20 μCi of radiotracer into the femoral vein. Rats were sacrificed by cardiotomy under isoflurane anesthesia at the indicated times. The target organ is removed and weighed, and the radioactivity is counted. Percentage of dose for each organ was calculated by comparing tissue counts to counts measured simultaneously at 1% of the initial dose (100-fold diluted aliquot of injected material). Brain regional distribution was measured in rats following intravenous injection of the radiotracer. Samples from different brain regions (cortex, striatum, hippocampus, cerebellum and hypothalamus) were dissected, weighed and counted. Calculate the percent dose / g for each sample by comparing the sample counts to the counts for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com