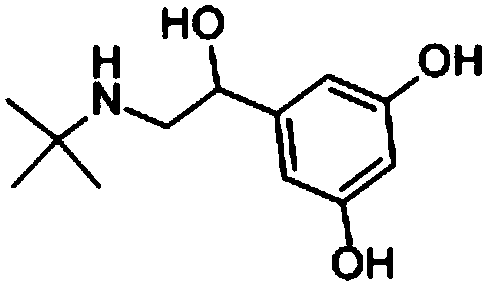
Method for the synthesizing terbutaline intermediates
A synthesis method and technology of terbutaline, applied in the field of medicine and chemical industry, can solve the problems of impurity content, inability to meet quality standard requirements of residue on ignition, insufficient income from input and output, and high price of Bambooterol, so as to avoid high energy The use of consuming operating conditions, the avoidance of the use of large amounts of solvents, the effect of easy degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
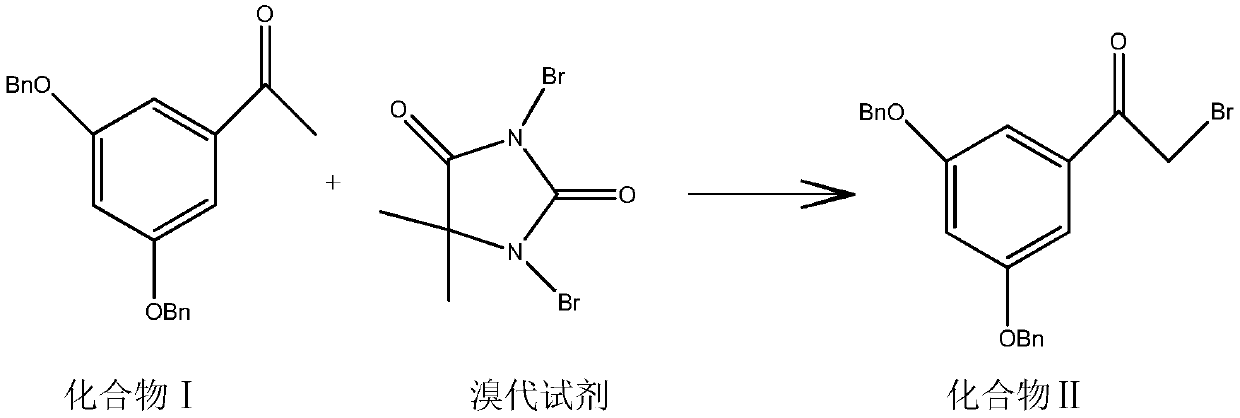
[0038] Add 100 g of the compound of formula I to 10 times (weight to volume ratio) of ethyl acetate, add 25 g of p-toluenesulfonic acid and 64 g of dibromohydantoin, replace with nitrogen for 3 times, and stir at 25°C under normal pressure for 5 hours in the dark. Suction filtration, the suction filtrate is placed in a reaction flask, washed three times, dewatered and dried, the suction filtrate is placed in a reaction flask and distilled under reduced pressure, concentrated to dryness, added 4 times (weight to volume) methanol and stirred for 35min, reduced to 10- Crystallize at 15°C for 1.5 h, and filter with suction to obtain 95 g of white crystalline solid with a yield of 76.76% and a purity of 99.37%.
[0039] 1 HNMR (400MHz, CDCl 3 ): δ (ppm): 4.415 (2H, s), 5.108 (4H, s), 6.878 (1H, m), 7.234-7.237 (2H, m), 7.284-7.469 (10, m).
[0040] 13 CNMR (100MHz, CDCl 3 ): δ (ppm): 31.22, 70.76, 108.09-108.19, 137.91-129.00, 138.50, 138.10, 160.43, 191.17.
Embodiment 2
[0042]Add 100 g of the compound of formula I to 5 times (weight to volume ratio) of ethyl acetate, add 20 g of trifluoroacetic acid and 80 g of dibromohydantoin, replace with nitrogen for 3 times, and stir at 25°C under normal pressure for 4 hours in the dark. Suction filtration, the suction filtrate is placed in a reaction flask, washed three times, dewatered and dried, the suction filtrate is placed in a reaction flask and distilled under reduced pressure, concentrated to dryness, added 5 times (weight to volume ratio) methanol and stirred for 30min, reduced to 10- Crystallize at 15°C for 2 hours, and filter with suction to obtain 89 g of white crystalline solid with a yield of 71.9% and a purity of 99.83%.
Embodiment 3
[0044] Add 100 g of the compound of formula I to 8 times (weight to volume ratio) of dichloromethane, add 18 g of p-toluenesulfonic acid and 70 g of dibromohydantoin, replace with nitrogen for 3 times, and stir for 5 hours at 25°C under normal pressure in the dark. Suction filtration, the suction filtrate is placed in a reaction flask, washed three times, dewatered and dried, the suction filtrate is placed in a reaction flask for vacuum distillation, concentrated to dryness, down to room temperature, add 3 times (weight to volume ratio) methanol and stir for 30min, Decrease to 10-15°C to crystallize for 2 hours, and filter with suction to obtain 93 g of white crystalline solid with a yield of 75.14% and a purity of 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com