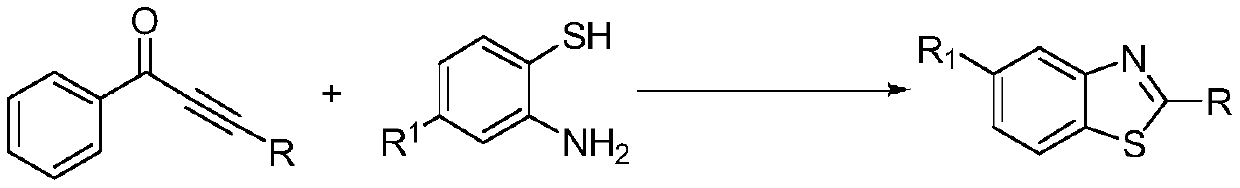
Method for preparing 1, 3-benzothiazole derivative through efficient catalysis of potassium tert-butoxide
A technology of potassium tert-butoxide and benzothiazole, which is applied in the field of efficient preparation of 1,3-benzothiazole derivatives, can solve the problems of complex substrate structure, long reaction time, cumbersome post-processing, etc. Stable, low-dose effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
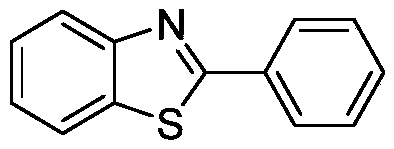
[0015] Preparation of 2-phenyl-1,3-benzothiazole with the following structural formula
[0016]
[0017] Add 0.103g (0.5mmol) 1,4-diphenyl-3-butyne-2-one, 0.0028g (0.025mmol) potassium tert-butoxide, 112μL (1.05mmol) anthranilin to the reaction flask, 1mL of tert-butanol, stirred and reacted at 50°C for 5 hours, stopped the reaction, added 5mL of distilled water, added 10mL of ethyl acetate to extract 3 times, rotatively evaporated the organic phase to remove ethyl acetate, and separated it with a silica gel column (the eluent was petroleum The volume ratio of ether and dichloromethane is 2:1 mixed solution), obtains 2-phenyl-1,3-benzothiazole, and its productive rate is 99%, and the spectral data of product is: 1 H NMR (400MHz, CDCl 3 )δ8.17-8.09(m,3H),7.95-7.88(m,1H),7.56-7.48(m,4H),7.44-7.36(m,1H); 13 C NMR (101MHz, CDCl 3 )δ168.98, 155.03, 135.97, 134.51, 131.90, 129.94, 128.49, 127.25, 126.12, 124.16, 122.55.
Embodiment 2
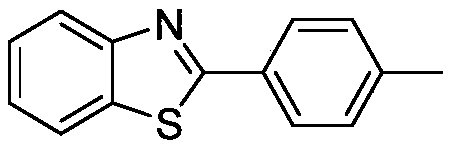
[0023] Preparation of 2-(4-tolyl)-1,3-benzothiazole with the following structural formula
[0024]
[0025] In this example, the 1,4-diphenyl-3-butane used in Example 1 was replaced with equimolar 1-phenyl-4-(4-methylphenyl)-3-butyn-2-one Alkyn-2-one, other steps are the same as in Example 1, to obtain 2-(4-tolyl)-1,3-benzothiazole, the yield is 99%, and the spectral data of the product is: 1 H NMR (400MHz, CDCl 3 )δ7.95(d, J=8.1Hz, 1H), 7.90-7.84(m, 2H), 7.76(d, J=8.0Hz, 1H), 7.39-7.33(m, 1H), 7.28-7.21(m ,1H),7.17(d,J=7.9Hz,2H),2.30(s,3H); 13 C NMR (101MHz, CDCl 3 )δ168.21, 154.21, 141.39, 134.98, 131.00, 129.71, 127.50, 126.23, 124.99, 123.07, 121.56, 21.52.
Embodiment 3
[0027] Preparation of 2-thienyl-1,3-benzothiazole with the following structural formula
[0028]
[0029] In this example, the 1,4-diphenyl-3-butyn-2-one used in Example 1 was replaced with equimolar 1-phenyl-4-thienyl-3-butyn-2-one, Other steps are the same as in Example 1 to obtain 2-thienyl-1,3-benzothiazole with a yield of 84%, and the spectral data of the product are: 1 H NMR (400MHz, CDCl 3 )δ8.06(d, J=8.2Hz, 1H), 7.84(d, J=8.0Hz, 1H), 7.65(d, J=3.5Hz, 1H), 7.52-7.46(m, 2H), 7.40- 7.34(m,1H),7.13(dd,J=5.1,3.8Hz,1H); 13 C NMR (101MHz, CDCl 3 )δ162.27, 154.62, 138.26, 135.63, 130.19, 129.52, 128.95, 127.33, 126.13, 123.88, 122.36.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com