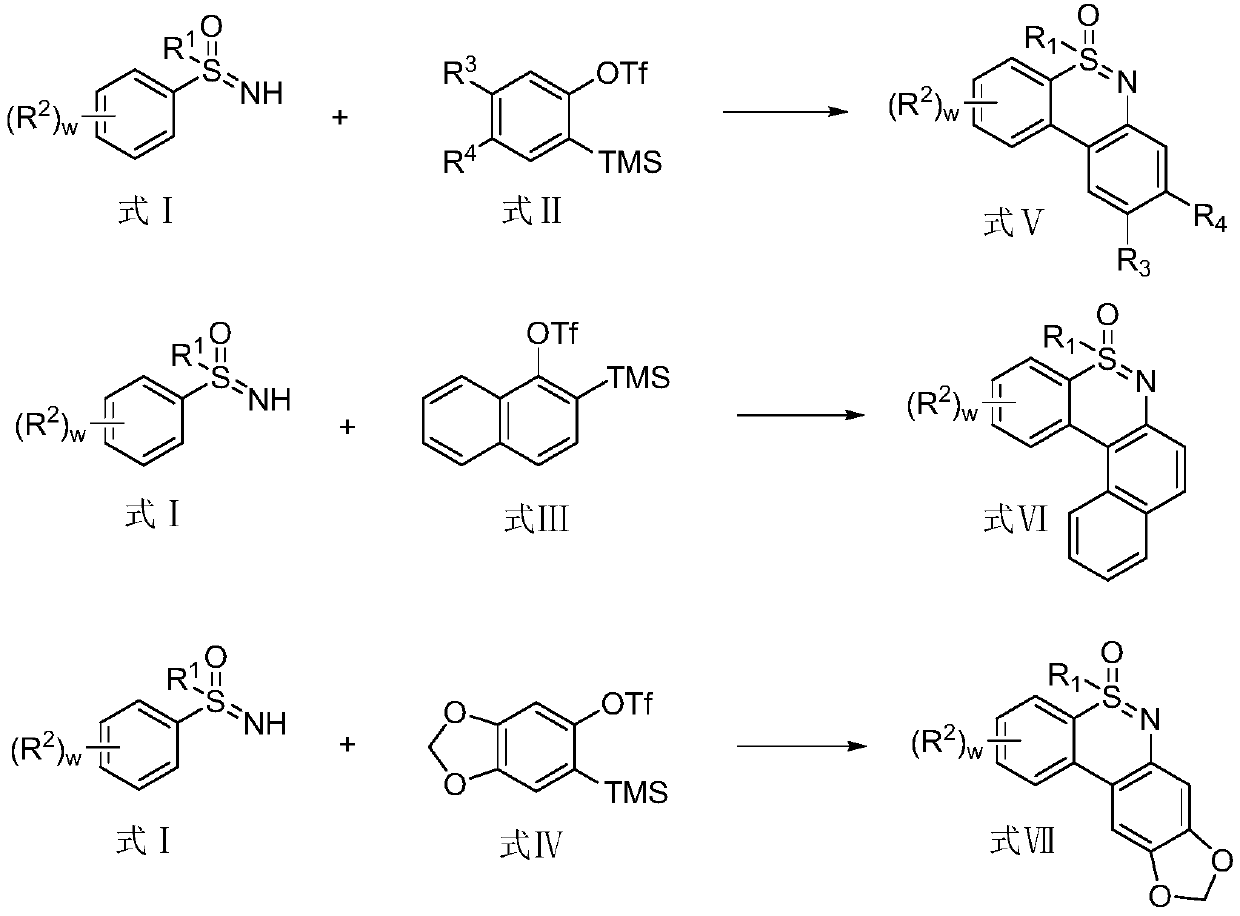
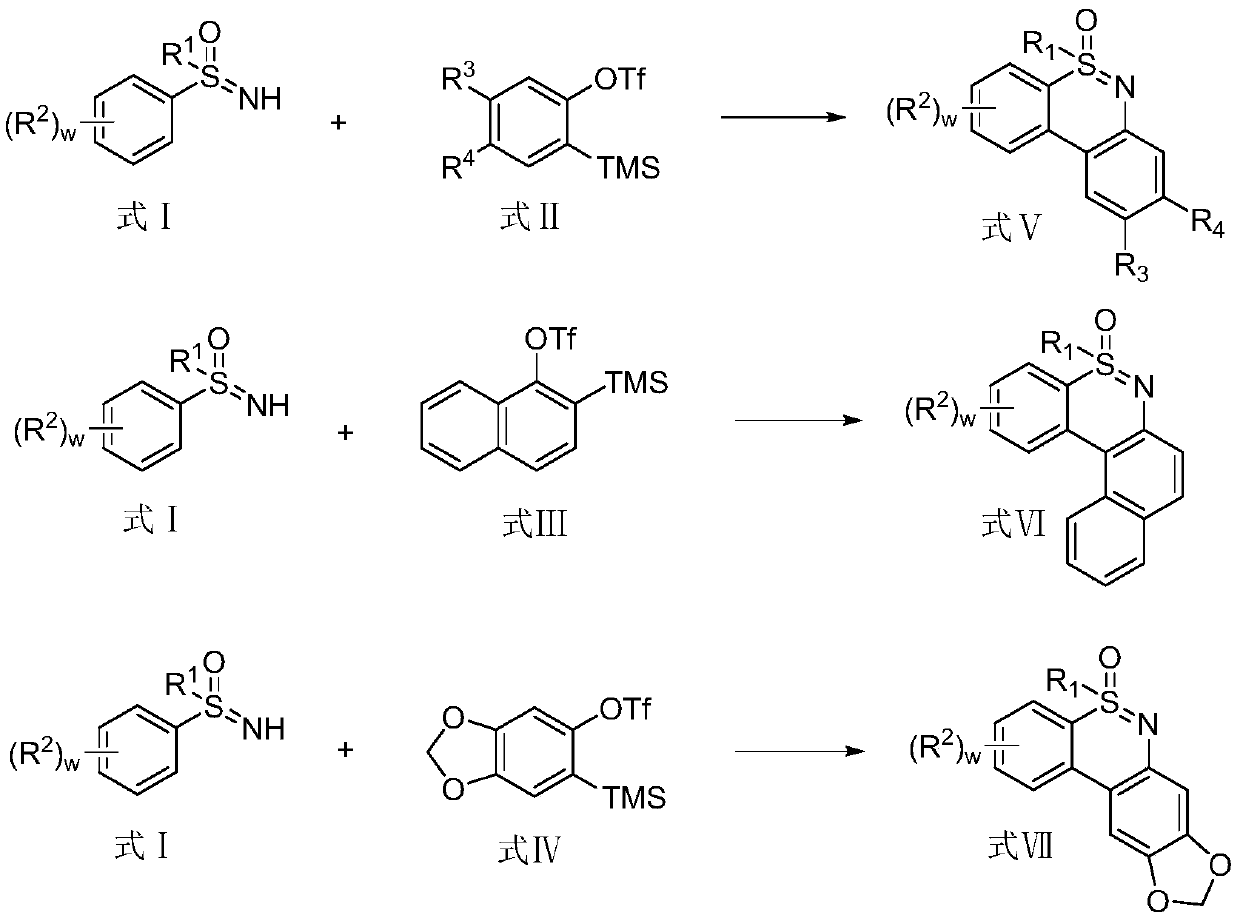
Synthesis method of 1, 2-benzothiazine compound
A technology for synthesizing benzothiazine and its synthesis method, which is applied in the field of synthesizing 1,2-benzothiazine compounds, which can solve the problems of unfriendly environment, small scope of application of substrates, expensive catalysts, etc., and shorten the synthesis route and reaction The effect of mild conditions and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
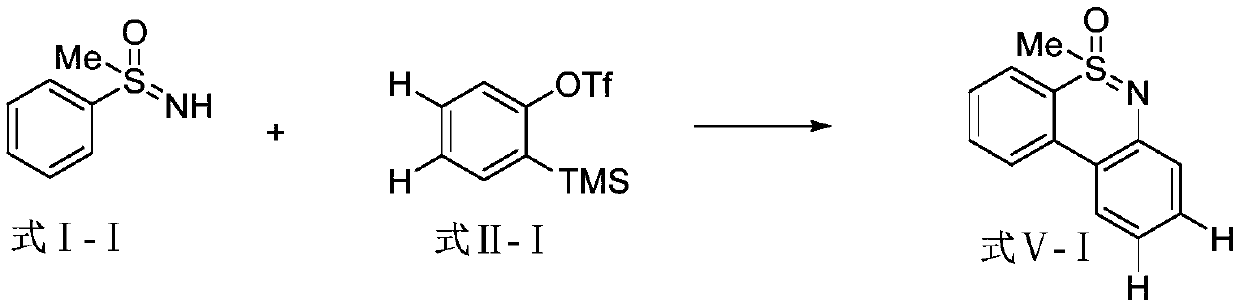
Embodiment 1
[0017]
[0018] Add 15.02mg (0.1mmol) of S-methyl-S-phenylsulfoximine shown in formula I-I, 4.49mg (0.02mmol) of palladium acetate, and 15.91mg (0.2mmol) of copper oxide in a 25mL pressure-resistant tube , 18.21mg (0.1mmol) 1-adamantanecarboxylic acid, 60.60mg (0.4mmol) cesium fluoride, 19.15mg (0.1mmol) cesium acetate, 2mL dioxane, 59.60mg (0.2mmol) shown in formula II-I 2-trimethylsilylphenyl trifluoromethanesulfonate, stirred at 110°C for 24h in a closed system protected by argon, cooled to room temperature after the reaction, extracted with ethyl acetate, combined the organic phases, and washed with anhydrous Na 2 SO 4 Drying, removal of ethyl acetate under reduced pressure, the crude product was separated by column chromatography (with a mixture of petroleum ether and ethyl acetate volume ratio of 1:3 as the eluent), to obtain 5-methanol shown in formula V-1 Dibenzo[c,e][1,2]thiazine-5-oxide, the isolated yield is 72%, and the structural characterization data are as ...
Embodiment 2
[0021]
[0022]In this example, replace the S-methyl-S-phenyl sulfoximine used in Example 1 with 4-methoxy-S-methyl-S-phenyl sulfoximine shown in equimolar formula I-II Sulfoximine, other steps are identical with embodiment 1, obtain the 4-methoxy-5-methyldibenzo [c, e] [1,2] thiazine-5-oxide compound shown in formula V-II , the isolated yield is 74%, and the structural characterization data are as follows:
[0023] 1 H NMR (600MHz, CDCl 3 )δ7.92(d, J=8.1Hz, 1H), 7.84(d, J=8.8Hz, 1H), 7.56(d, J=2.1Hz, 1H), 7.37(t, J=8.2Hz, 1H) ,7.23(d,J=8.1Hz,1H),7.10(d,J=8.7Hz,1H),7.05(t,J=7.0Hz,1H),3.96(s,3H),3.45(s,3H) ; 13 C NMR (151MHz, CDCl 3 )δ163.00, 143.45, 136.52, 130.84, 126.33, 124.82, 123.51, 120.54, 117.58, 117.15, 115.43, 106.92, 55.71, 45.57; HRMS (ESI) m / z: C 14 h 13 NOS[M+Na] + The theoretical value is 282.0599, and the measured value is 282.0560.
Embodiment 3
[0025]
[0026] In this example, replace the S-methyl-S-phenyl sulfoxide used in Example 1 with 4-methyl-S-methyl-S-phenyl sulfoximine shown in equimolar formula I-III imine, other steps are the same as in Example 1 to obtain 4-methyl-5-methyldibenzo[c,e][1,2]thiazine-5-oxide shown in formula V-IIII, which The isolated yield is 73%, and the structural characterization data are as follows:
[0027] 1 H NMR (600MHz, CDCl 3 )δ8.01-7.97(m,2H),7.80(d,J=8.1Hz,1H),7.39-7.35(m,2H),7.24(d,J=7.9Hz,1H),7.07(t,J =7.5Hz,1H),3.49(s,3H),2.54(s,3H); 13 C NMR (151MHz, CDCl 3 )δ142.64, 142.05, 133.06, 129.56, 127.99, 123.77, 122.99, 122.87, 122.44, 121.27, 119.66, 116.31, 76.24, 76.03, 75.82, 43.98, 21.12; HRMS (ESI) m / z 14 h 13 NOS[M+Na] + The theoretical value is 266.0610, and the measured value is 266.0612.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com