Synthesis method of 3-(7-hydroxy-1-oxoisoindole-2-yl) piperidine-2, 6-dione
A synthesis method and isoindole technology, applied in the direction of organic chemistry and the like, can solve the problems of easy ring opening and severe reaction of the synthesis method, and achieve the effects of easy operation, mild reaction conditions, and cheap and easily available raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
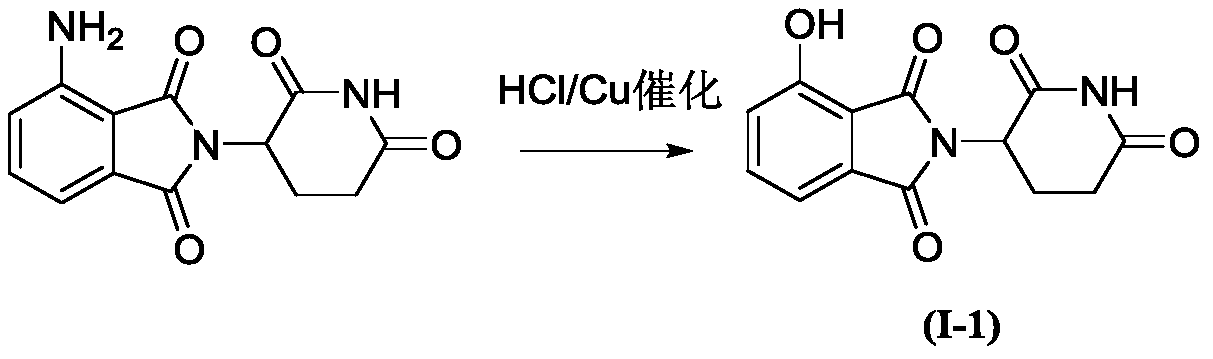
[0024] Example 1: Synthesis of 2-(2,6-dioxopiperidin-3-yl)-4-hydroxyisoindole-1,3-dione:
[0025]
[0026] Under ice-bath conditions, add 4-amino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (2.73 g, 1mmol), and stirred for 1h. Then an appropriate amount of copper powder (0.013g, 0.2mmol) and NaNO 2 (0.69g, 1mmol) was slowly added to the mixture, and after stirring for 30min, the temperature was raised to 80°C for 1h. After the reaction, it was extracted with ether and dried over anhydrous magnesium sulfate. Most of the solvent was spun off, and purified by silica gel column chromatography, eluting with petroleum ether / ethyl acetate (V / V=20 / 1) to obtain compound I-1 (2.47 g). Yield: 90%.
[0027] 1 H NMR (400MHz, CDCl 3 )δ9.20(s,1H),7.78(dd,1H),7.46(t,1H),7.11(dd,1H),5.50(t,1H),2.61(td,2H),2.23(m,2H ).
Embodiment 2
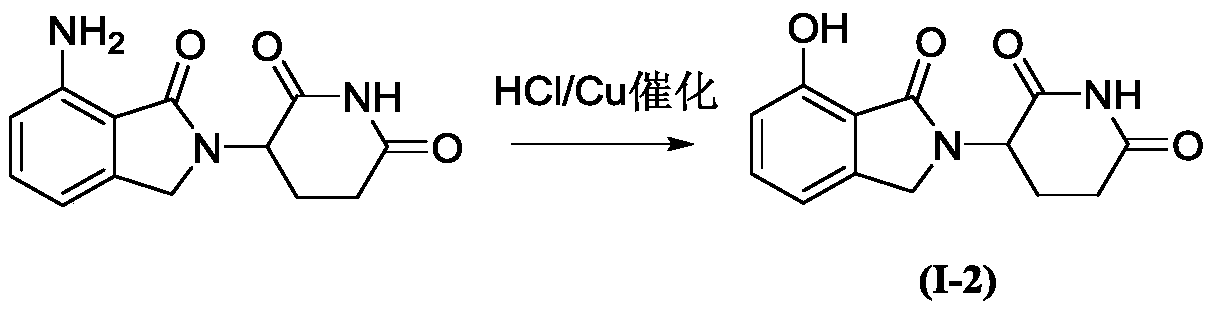
[0028] Example 2: Synthesis of 3-(7-hydroxyl-1-oxoisoindol-2-yl)piperidine-2,6-dione:
[0029]
[0030] Under ice-bath conditions, add 4-amino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (2.73 g, 1mmol), and stirred for 1h. Then an appropriate amount of copper powder (0.013g, 0.2mmol) and NaNO 2 (0.69g, 1mmol) was slowly added to the mixture, and after stirring for 30min, the temperature was raised to 80°C for 1h. After the reaction, it was extracted with ether and dried over anhydrous magnesium sulfate. Most of the solvent was spun off, and purified by silica gel column chromatography, eluting with petroleum ether / ethyl acetate (V / V=20 / 1) to obtain compound I-2 (2.34 g). Yield: 90%.
[0031] 1 H NMR (400MHz, CDCl 3 )δ9.09(s,1H),7.21(m,1H),6.98(ddt,1H),6.87(dd,1H),4.44(t,1H),4.30(d,2H),2.58(qt,2H ), 2.07(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com