Cell preparation capable of treating allergic rhinitis
A technology for allergic rhinitis and cell preparation, applied in allergic diseases, animal cells, vertebrate cells, etc., can solve problems such as affecting patients' study and work, and obvious general discomfort.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1, Preparation of Umbilical Cord Wharton Section Mesenchymal Stem Cell Preparation
[0056] 1. Obtaining the fetal umbilical cord
[0057] The isolated umbilical cords of full-term newborns without congenital diseases were obtained; the mothers had no infectious diseases such as hepatitis, syphilis, and AIDS, and the mothers and their families gave informed consent to the use of the umbilical cords in the experimental research.
[0058] 2. Obtaining mesenchymal cells from the Wharton section of the umbilical cord
[0059] 1. In the aseptic laboratory bench, the umbilical cord is repeatedly rinsed with saline to remove residual blood. Cut the umbilical cord into small pieces of 2-3cm with sterile surgical instruments, cut the umbilical cord longitudinally, remove the umbilical artery, umbilical vein and amniotic membrane, take the Wharton area, and cut it into 0.5-1mm 3 Little pieces left and right.
[0060] 2. Obtain primary umbilical cord mesenchymal cells b...
Embodiment 2
[0072] Embodiment 2, identification of P3 cell preparation
[0073] The test product is the P3 cell preparation prepared in Example 1.
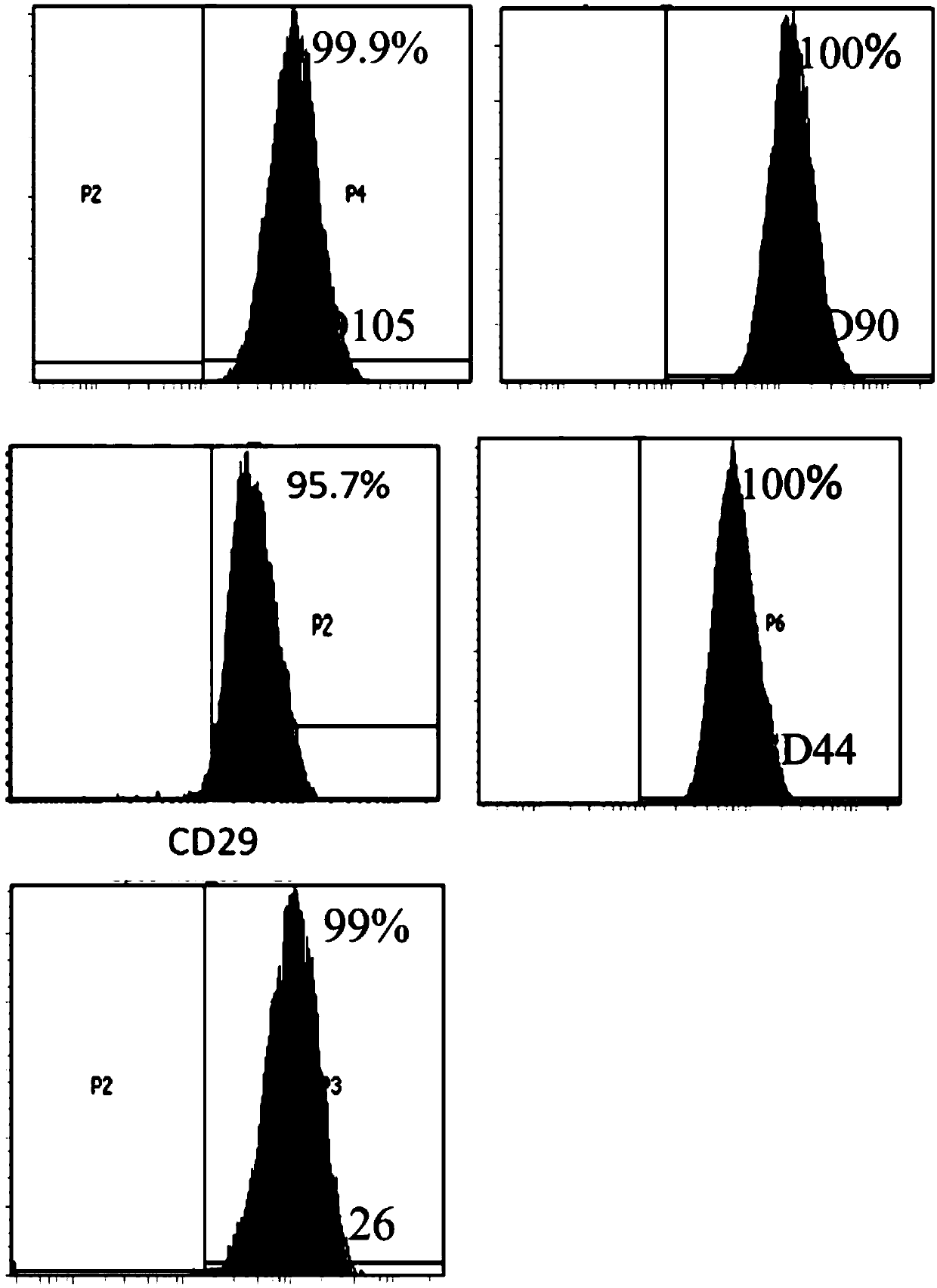
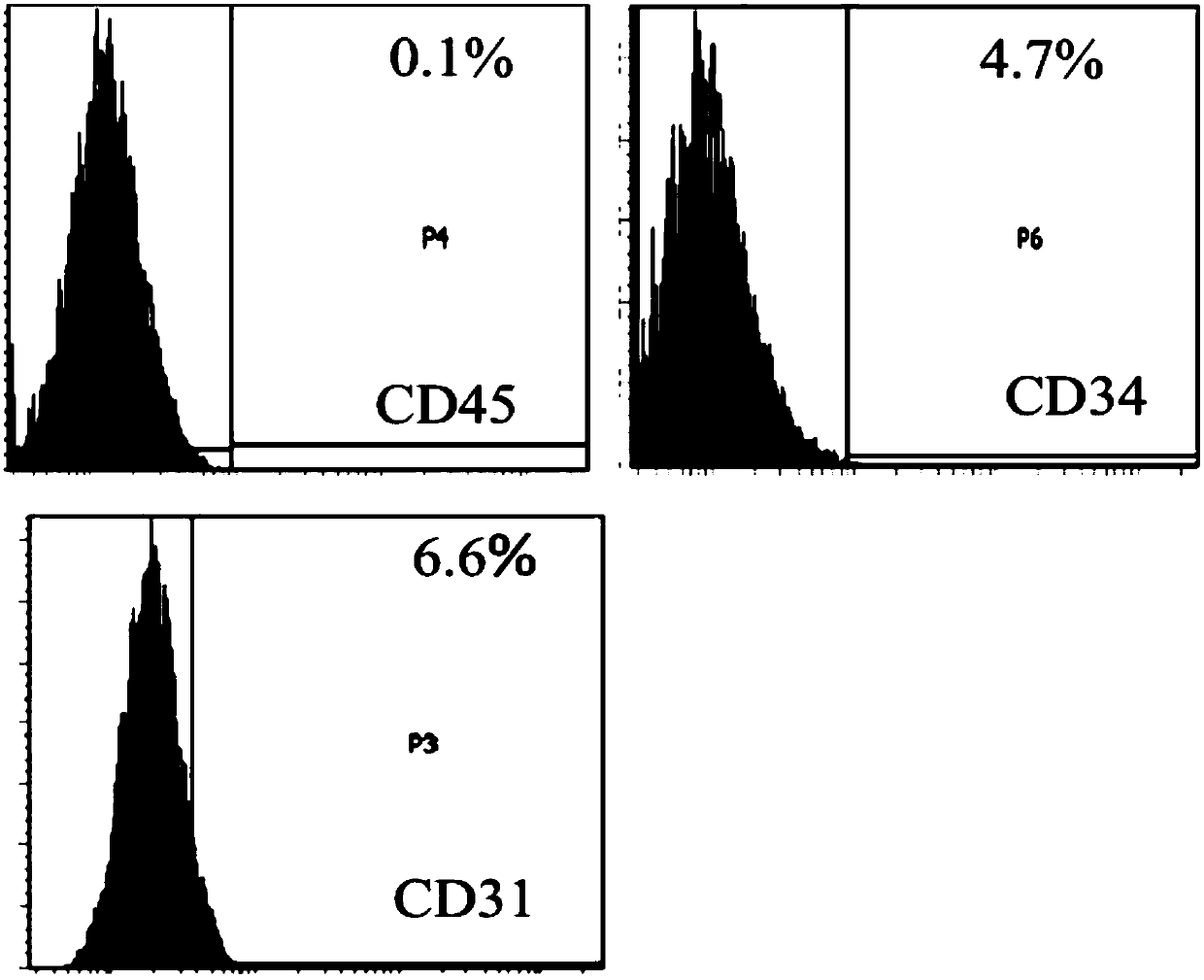
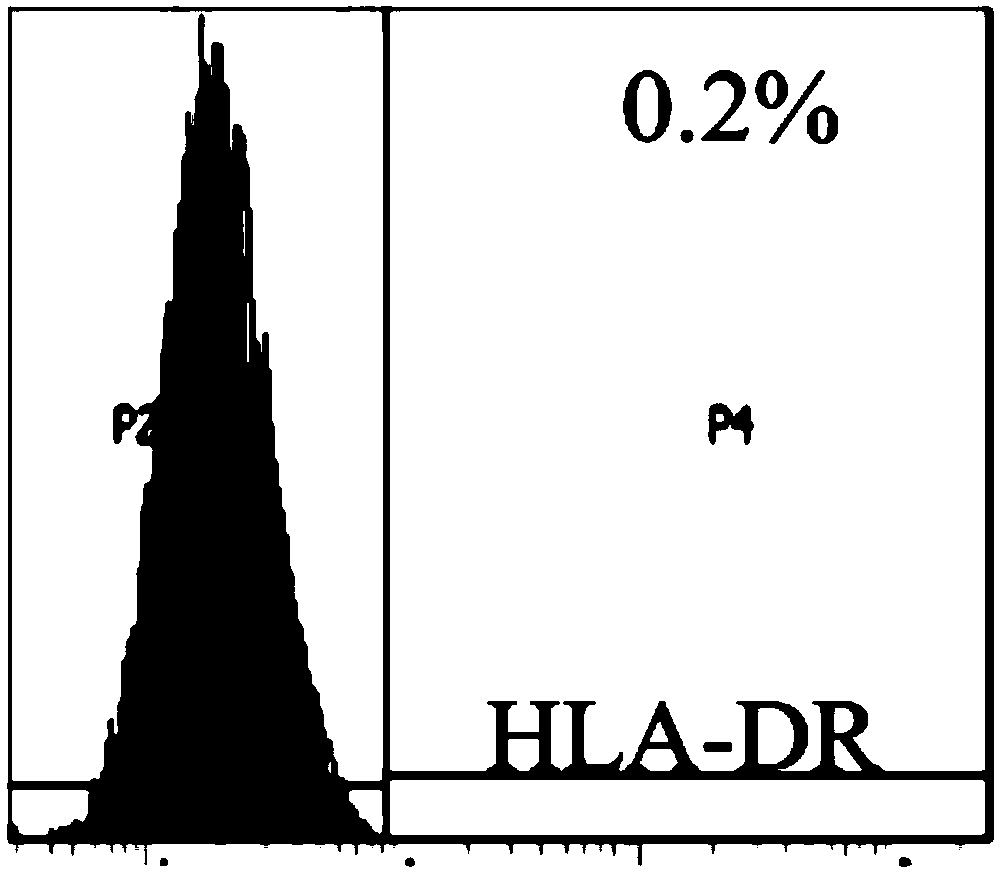
[0074] Use flow cytometry to detect the expression of CD29, CD44, CD90, CD105, CD126, CD31, CD45, CD34 and HLA-DR in the test product. The specific steps are: after the cells are incubated with the corresponding antibodies, the excess antibodies are washed away , resuspend the cells with PBS buffer, and use the LSR Fortessa instrument of BD Company to detect the positive rate of each index.
[0075] For the results of markers of mesenchymal stem cells (CD29, CD44, CD90, CD105 and CD126), see figure 1 . For the results of hematopoietic stem cell and endothelial cell markers (CD31, CD45 and CD34), see figure 2 . For the results of transplant rejection-related markers (HLA-DR), see image 3 . The results show that the test product highly expresses markers of mesenchymal stem cells (both positive rates are above 95%), lowly expresses marke...
Embodiment 3
[0076] Embodiment 3, the therapeutic effect of P3 cell preparation on allergic rhinitis
[0077] Antigen adjuvant suspension: ovalbumin 1mg / ml, aluminum hydroxide 2mg / ml, and the balance is normal saline.
[0078] Egg protein solution: 0.1 mg / ml egg protein, and the balance is normal saline.
[0079] 1. Model preparation and administration
[0080] Allergic rhinitis model induced by sensitization of rats with egg protein and aluminum hydroxide gel.
[0081] SD rats, half male and half male, weighing about 250g, were first adapted to feeding for a week (clean animal breeding room, free intake of water), and then grouped as follows (12 rats in each group):
[0082] Cell therapy group: on the first day of the test, inject the antigen adjuvant suspension (one point on the plantar of each hindlimb, three points in the abdominal cavity, a total of 5 points, each point injecting 0.2ml); on the seventh day of the test, inject the antigen adjuvant Suspension (one point on the planta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com