Low-temperature, high-efficiency and energy-saving low-pressure decomposition process for urea production
A low-pressure, urea technology, used in the preparation of products, urea derivatives, and organic compounds, etc., can solve the problems of high consumption of low-pressure decomposition steam, heavy load in the recovery section, and high consumption of decomposition steam, and achieve good waste heat utilization effect and equipment. Longer life and less steam usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
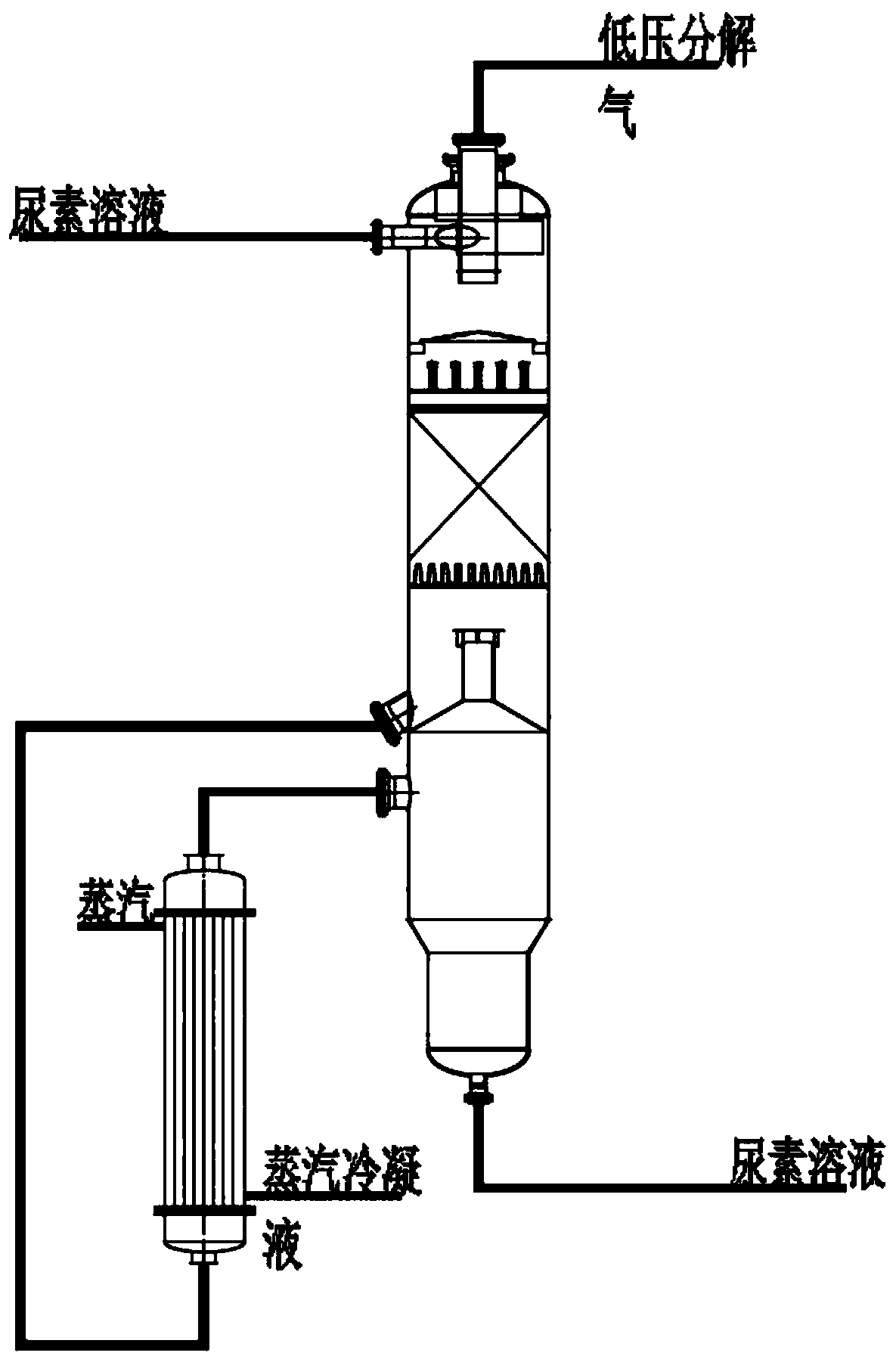
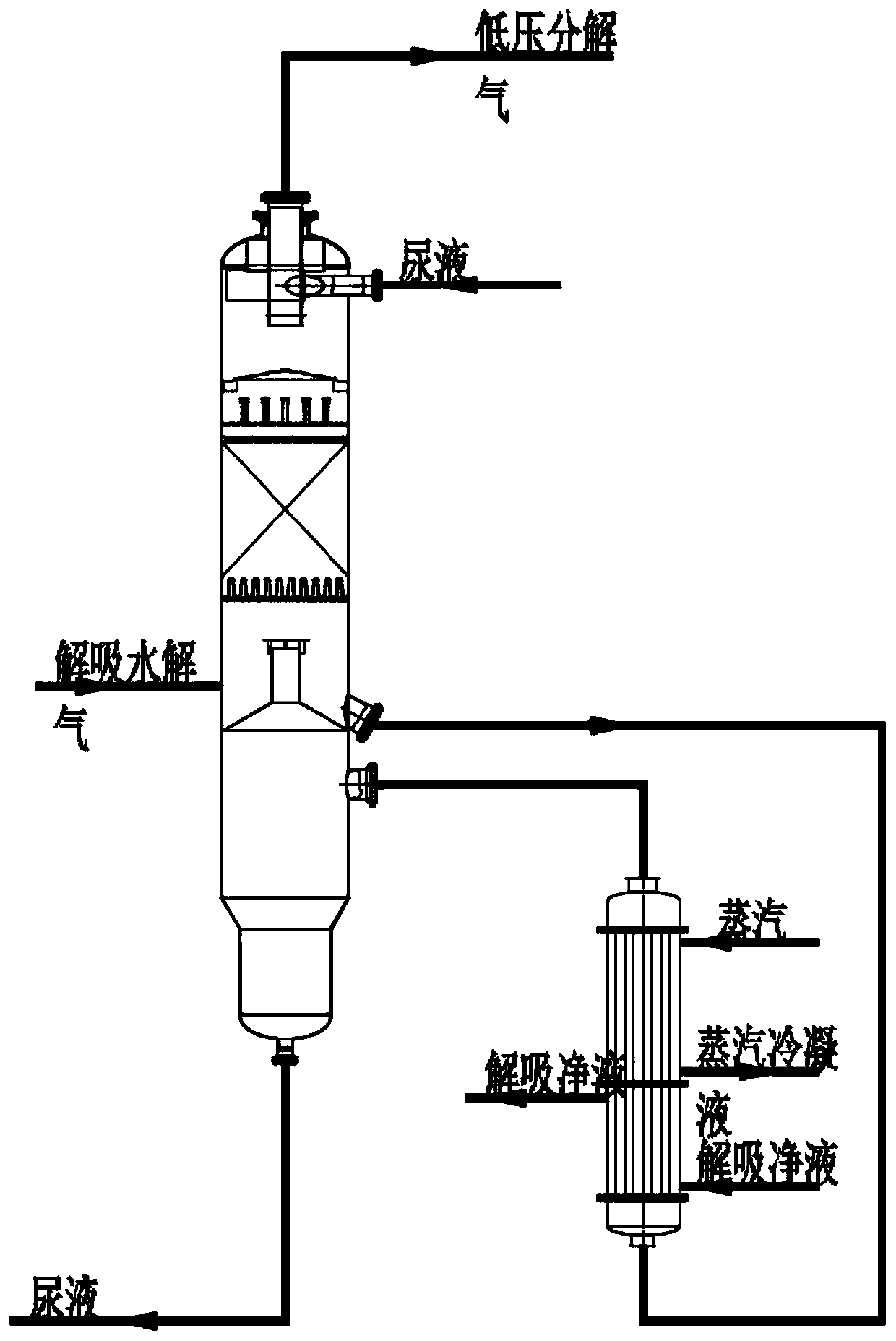
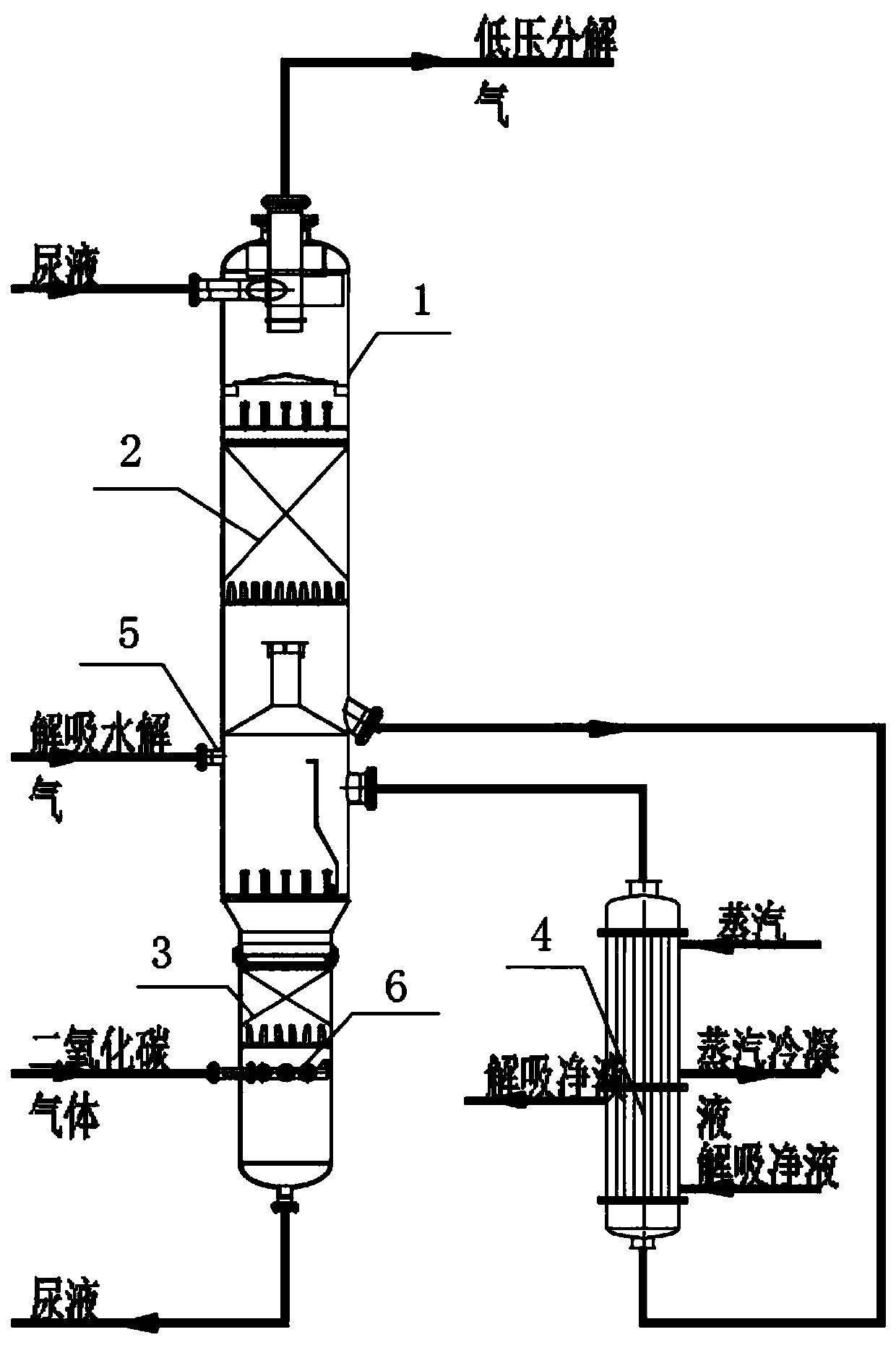
[0039] A low-temperature, low-pressure, high-efficiency decomposition process for urea production, including the following process:
[0040] (1) Heat exchange of packing material in the upper section:
[0041] From the reaction mixture (total mass percentage sum is 100%) that contains urea 61.41%, 4.61% ammonium carbamate, 27.23% water and 6.75% free ammonia after the decompression of medium pressure decomposition tower, enters the low pressure decomposition tower top The packing section is used for mass and heat exchange under the condition of 0.25MPa pressure;
[0042] (2), low-pressure decomposition heater heating:
[0043] The solution exiting the upper packing section enters from the bottom of the low-pressure decomposition heater, and is heated to 130°C to 135°C by the second-stage low-pressure decomposition heater, and most of the ammonium carbamate in the solution is decomposed into NH 3 and CO 2 , free ammonia is evaporated;
[0044] (3), desorption hydrolysis gas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com