Nickel-cobalt-aluminum hydroxide precursor, preparation method thereof, anode material and battery
A hydroxide, nickel-cobalt-aluminum technology, applied in the field of electrochemistry, can solve the problems of small particles, poor crystallinity, uneven particle size, etc., and achieve the effect of uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
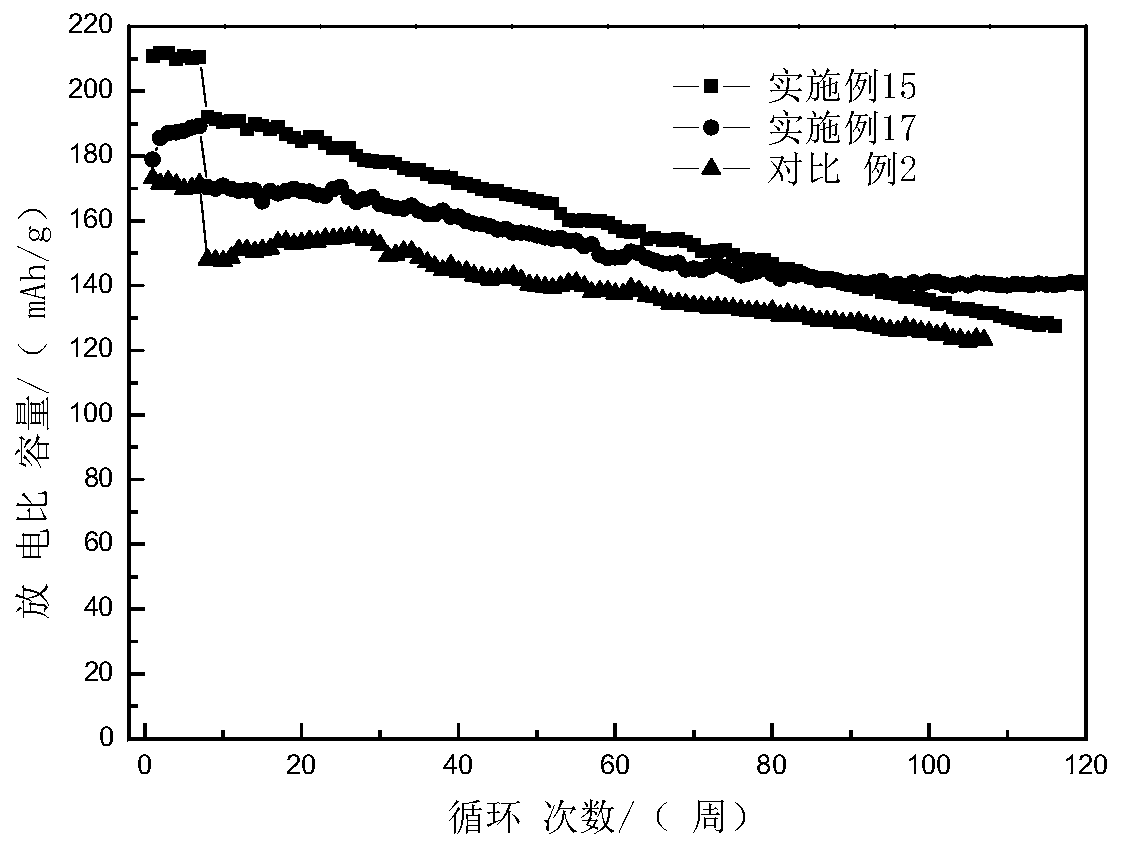
Examples
preparation example Construction
[0095] The invention provides a method for preparing a nickel-cobalt-aluminum hydroxide precursor, comprising:
[0096] In the embodiment of the present invention, a surfactant is added during the preparation of the nickel-cobalt-aluminum hydroxide precursor by the chemical co-precipitation method to promote the particle growth of the nickel-cobalt-aluminum hydroxide precursor, especially the primary particle growth, so that the The morphology of the nickel-cobalt-aluminum hydroxide precursor tends to be spherical. At the same time, it can make the crystal grow in a certain direction, increase the crystallinity, improve the density, and then improve the performance of the precursor, and the surfactant will not affect the precursor. The layered structure ensures the performance of the precursor.
[0097] Specifically, the surfactant includes at least one of anionic surfactants, nonionic surfactants and zwitterionic surfactants. Anionic surfactants are ionized in water, and the...
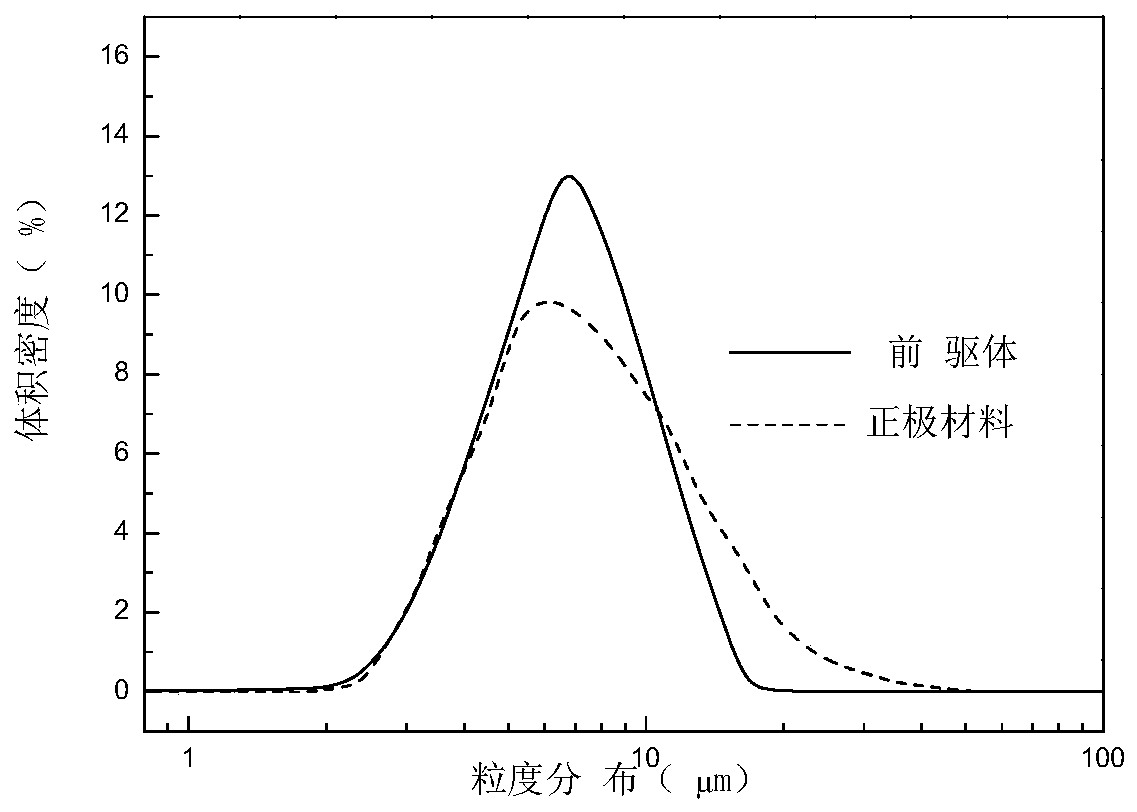
Embodiment 1
[0191] Take 94.63gNiSO 4 ·6H 2 O, 18.98gCoSO 4 ·7H 2 O, 7.50gAl 2 (SO4) 2 18H 2 O is made into a metal salt solution with a nickel-cobalt-aluminum concentration of 1.5 mol / L. Prepare the first mixed solution with NaOH concentration of 3 mol / L, ammonia concentration of 0.6 mol / L, and sodium dodecylbenzenesulfonate of 6 g / L. A second mixed solution with an ammonia concentration of 0.3 mol / L and a sodium dodecylbenzenesulfonate of 3 g / L was prepared.
[0192] The initial pH of the second mixed solution was 11.12, and the temperature was 50°C. Then inject the metal salt solution and the first mixed solution into the reaction kettle containing the second mixed solution respectively, adjust the flow rate of the first mixed solution to control the pH of the reaction system=10.00±0.03, the temperature is 50°C, and the stirring speed is 540r / min , the reaction time is 3h, and the slurry of nickel-cobalt-aluminum hydroxide is obtained. The slurry is naturally cooled and left to ...
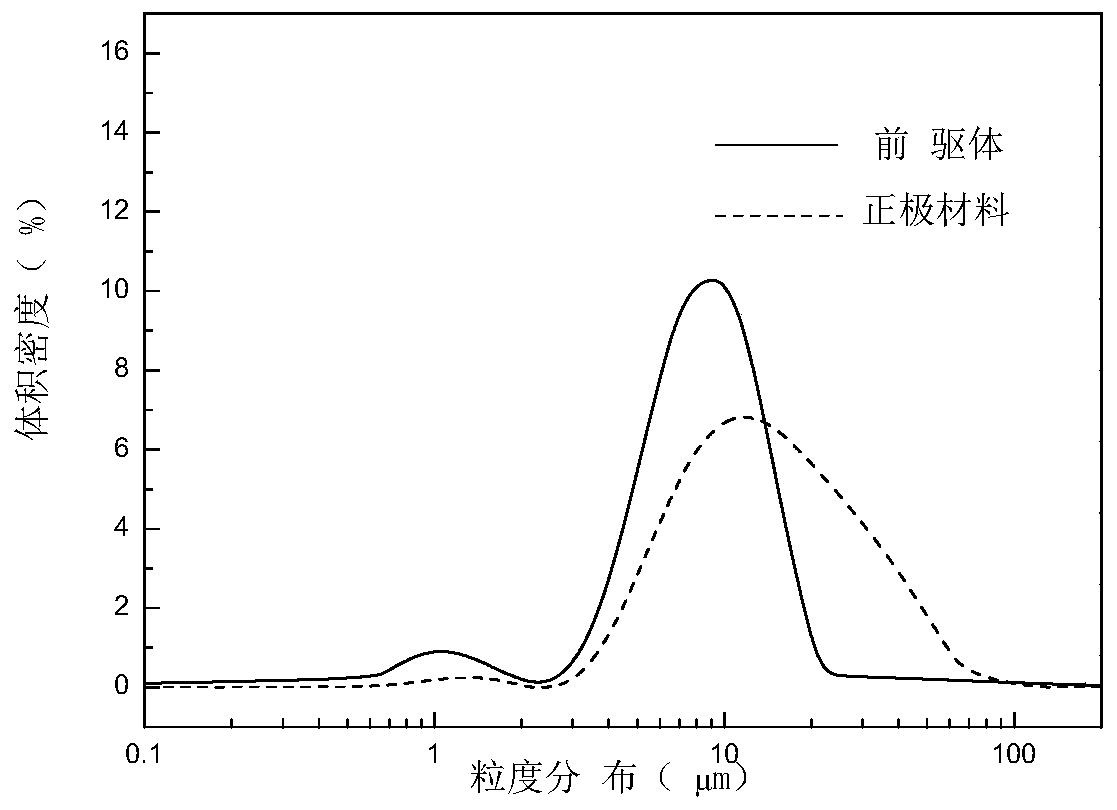
Embodiment 2
[0195] Take 210.28gNiSO 4 ·6H 2 O, 42.17gCoSO 4 ·7H 2 O is formulated into a metal salt solution with a nickel-cobalt concentration of 1.6 mol / L. Take 4.10g NaAlO 2 , 8.00g of NaOH is prepared into aluminum solution, the concentration of aluminum solution is 0.17mol / L. Prepare NaOH concentration as 2mol / L, ammonia concentration as 0.6mol / L, sodium dodecylbenzenesulfonate as the first mixed solution of 6g / L, prepare ammonia concentration as 0.3mol / L, dodecylbenzenesulfonic acid Sodium is the second mixed solution of 3g / L.
[0196] The initial pH of the second mixed solution was 11.20, and the temperature was 50°C. Then the metal salt solution, the aluminum solution, and the first mixed solution are injected into the reaction kettle containing the second mixed solution respectively, and the flow rate of the first mixed solution is adjusted to control the pH of the reaction system=10.00±0.03, the temperature is 50° C., and the stirring speed is 900r / min, the reaction time ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com