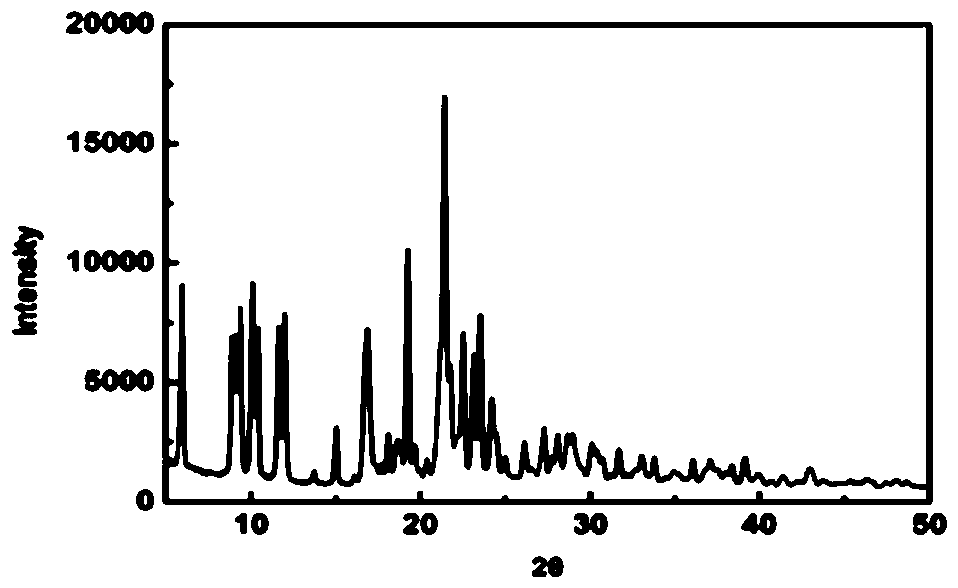
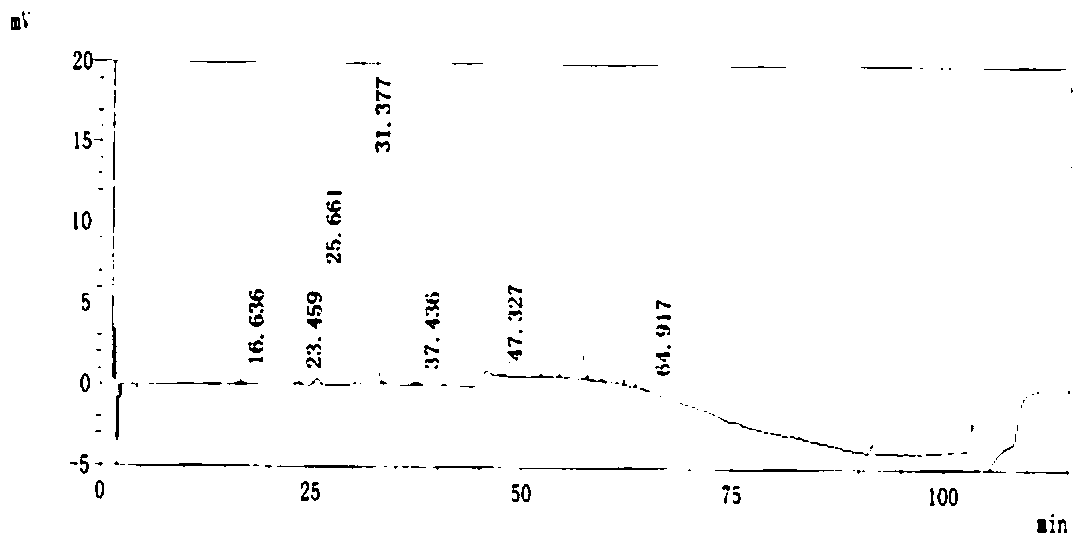
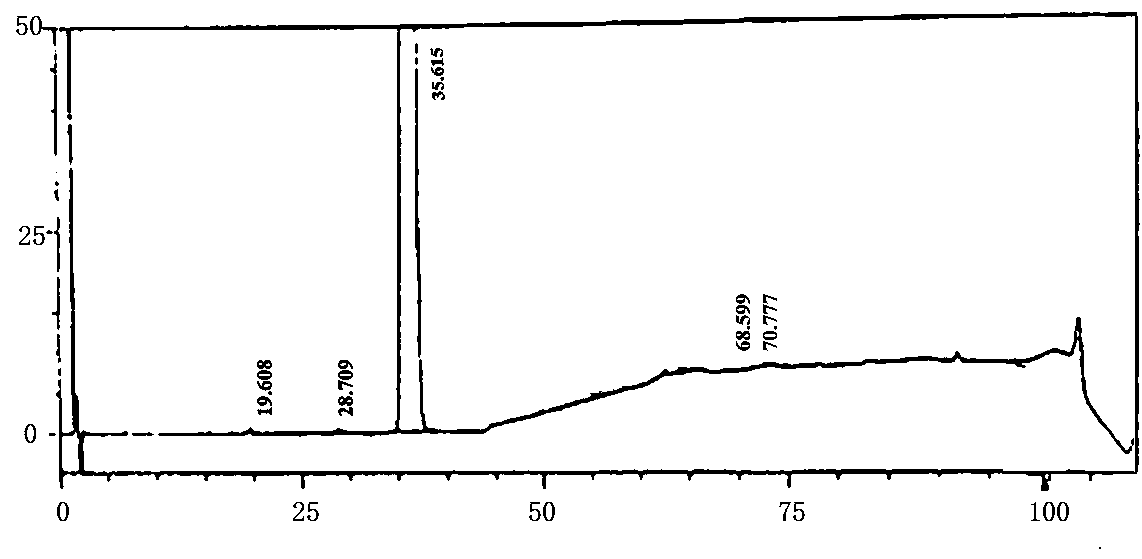
Preparation method of crystal form I atorvastatin calcium
A technology of atorvastatin calcium and atorvastatin, which is applied in the fields of organic chemistry and organic chemistry, and can solve problems such as the crystal form and purity of atorvastatin calcium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The invention provides a preparation method of I crystal form atorvastatin calcium, which comprises the following steps:
[0036] S1. Preparation of atorvastatin ester
[0037]Atorvastatin intermediate L1, (4R,6R)-2-[6-[2-[2-(4-fluorophenyl)-5-isopropyl ester-3-phenyl-4-(phenylamine Formyl)pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]tert-butyl acetate (hereinafter referred to as "intermediate L1"), purity testing The content of the main peak shall not be less than 98%, the single impurity shall not be higher than 0.3%, and the total impurity shall not be higher than 1.0%. The protection of the isopropyl group is removed under acidic conditions. The reaction process is as follows:
[0038]
[0039] In some specific embodiments of the present invention, the atorvastatin intermediate L1 is dissolved in alcohol, and the alcohol is selected from one or more of methanol, ethanol, n-propanol, or isopropanol, preferably methanol.
[0040] In some specific embodiments o...
Embodiment 1
[0063] Put 120g of intermediate L1 and 600ml of methanol into a 2000ml reaction kettle, then add the prepared hydrochloric acid solution (142g of purified water, 16.4g of hydrochloric acid), slowly raise the temperature to 30°C, react for 5 hours, TLC spot plate monitoring, raw material point Disappearance is the end point of the reaction.
[0064] After the reaction in the previous step is complete, add 10% NaOH solution (sodium hydroxide 14.2g, water 127.8g) to the reaction solution, react at 30°C for 5 hours, monitor with TLC, and the disappearance of the atorvastatin ester point is the reaction end.
[0065] After the reaction in the previous step is complete, directly add calcium acetate aqueous solution (17.8 g of calcium acetate; 240 g of water), stir and react at 20° C. for 2 hours, and centrifuge to obtain the crude atorvastatin calcium wet product. Dry in a hot air circulation oven at 50°C to obtain the crude atorvastatin calcium dry product.
[0066] Add the crude...
Embodiment 2
[0077] Put 120g of intermediate L1 and 600ml of methanol into a 2000ml reaction kettle, then add the prepared hydrochloric acid solution (142g of purified water, 16.4g of hydrochloric acid), slowly raise the temperature to 30°C, react for 5 hours, TLC spot plate monitoring, raw material point Disappearance is the end point of the reaction.
[0078] After the reaction in the previous step is complete, add 10% NaOH solution (sodium hydroxide 14.2g, water 127.8g) to the reaction solution, react at 30°C for 5 hours, monitor with TLC, and the disappearance of the atorvastatin ester point is the reaction end.
[0079] After the reaction in the previous step is complete, directly add calcium acetate aqueous solution (17.8 g of calcium acetate; 240 g of water), stir and react at 20° C. for 2 hours, and centrifuge to obtain the crude atorvastatin calcium wet product. Dry in a hot air circulation oven at 50°C to obtain the crude atorvastatin calcium dry product.
[0080] Add the crude...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com